Jul 18 2016
A multi-disciplinary research, spanning several years has revealed that a new 3D sponge composed of carbon nanotubes was capable of promoting nerve fiber growth, linking separated neural explants and offering a practical reconnection.
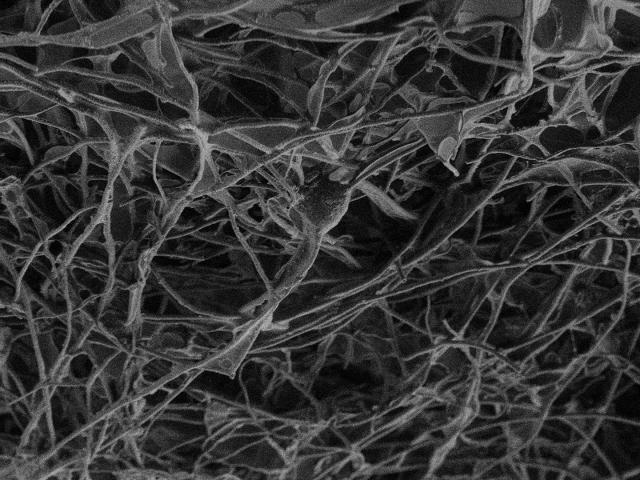 This image shows nanotubes scaffold. (Credit: SISSA/UNITS)
This image shows nanotubes scaffold. (Credit: SISSA/UNITS)
This research was coordinated by the International School for Advanced Studies (SISSA) in Trieste, in partnership with and the University of Trieste and the University of Rome Tor Vergata. Through their research, the team also noticed the material’s biocompatibility in vivo, showing that implanting it into a small rodent’s brain does not cause a noticeable immune response or large scars. The findings have been published in Science Advances. The paper explains that the material could probably be used in biomedical implementation and could be assessed for prosthetic nervous system applications.
Under the microscope, it looks like a knotted tangle of tubes. It was initially studied by Maurizio De Crescenzi's team at the University of Rome Tor Vergata for cleaning up spilled hydrocarbons in the sea.
Laura Ballerini, Professor, SISSA
It was Maurizio Prato's hunch, however, that motivated them to explore the option of using such a material to nerve tissue.
In the current research, Ballerini and her team initially analyzed the reaction of the material to nerve tissue in vitro.
We explanted two spinal cord segments and cultured them together but separated by 300 microns. In those conditions, without any scaffolds reconstructing the space between the two explants, we observed growth of nerve fibers which extended in a straight bundles in any direction, but not necessarily towards the other tissue. If we insert a small piece of the carbon sponge into the space between the two, however, we see dense growth of nerve fibers that fill the structure and intertwine with the other sample.
Sadaf Usmani, PhD student, SISSA
Carbon nanotubes scaffold
This Video Shows Nanotubes Scaffold In A 3-D Animation. (Photo credit: SISSA/UNITS)
"Observing fiber reaching the contralateral explant is not enough, however," points out University of Trieste researcher and one of the authors of the study, Denis Scaini. "You have to show that there is a functional connection between the two populations of neurons." For this, SISSA Professor, David Zoccolan and his team's contribution were crucial. "With signal analysis techniques they had already developed, we were able to demonstrate two things: first, that spontaneous nervous activity in the two samples was actually correlated, indicating a connection, that was not there when the sponge was absent, and second, that by applying an electrical signal to one of the samples, the activity of the second sample could be triggered, but only when the nanotubes were present."
Tests for Biocompatibility
The lab results were highly positive. But this was not enough for Ballerini and her colleagues. "In order to continue to invest additional energy and resources to the study for potential applications, is crucial to test if the material is accepted by living organisms without negative consequences," says Ballerini.
To conduct these tests, Ballerini's team associated closely with SISSA Post-Doc researcher, and member of Zoccolan's team, Federica Rosselli.
We implanted small portions of the material into the brain of healthy rodents. After four weeks, we observed that the material was well tolerated. There were limited scars, as well as low immune responses, some biological indicators even showed that there could be positive implications. There was also a progressive invasion of neurons within the sponge. The rats were vital and healthy during the entire four weeks.
Sadaf Usmani, PhD student, SISSA
"In conclusion," says Ballerini, "the excellent results at the structural and functional level in vitro and in vivo showed biocompatibility are encouraging us to continue this line of research. These materials could be useful for covering electrodes used for treating movement disorders like Parkinson's because they are well accepted by tissue, while the implants being used today become less effective over time because of scar tissue. We hope this encourages other research teams with multidisciplinary expertise to expand this type of study even further."