Sep 15 2016
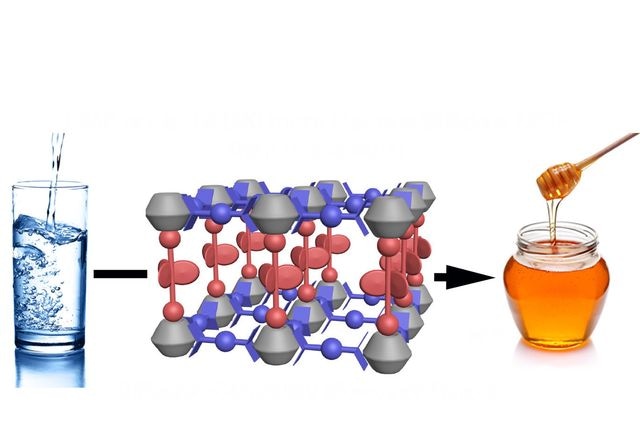 A fluid with a viscosity like water enters UCLA-R3, where its viscosity at the nanoscale becomes like honey. (Credit: Xing Jiang, Miguel García-Garibay/UCLA Chemistry and Biochemistry)
A fluid with a viscosity like water enters UCLA-R3, where its viscosity at the nanoscale becomes like honey. (Credit: Xing Jiang, Miguel García-Garibay/UCLA Chemistry and Biochemistry)
Nanoscience researchers at UCLA have established that a fluid that behaves similar to water in daily life turns as heavy as honey when trapped in a nanocage of a porous solid, providing new insights into the way matter acts in the nanoscale world.
We are learning more and more about the properties of matter at the nanoscale so that we can design machines with specific functions.
Miguel García-Garibay, UCLA
García-Garibay is the dean of the UCLA Division of Physical Sciences and a professor of chemistry and biochemistry.
The study is published in the ACS Central Science journal.
A nanometer is roughly 1/20,000 the diameter of a human hair and below 1/1,000 the size of a red blood cell. Despite years of research by scientists from all parts of the world, the extremely small size of matter at the nanoscale has made it difficult to discover how motion functions at this scale.
This exciting research, supported by the National Science Foundation, represents a seminal advance in the field of molecular machines. It will certainly stimulate further work, both in basic research and real-life applications of molecular electronics and miniaturized devices. Miguel Garcia-Garibay is among the pioneers of this field and has a very strong record of high-impact work and ground-breaking discoveries.
Eugene Zubarev, Program Director, National Science Foundation
Potential uses for complex nanomachines that could be a lot smaller than a cell include: putting a pharmaceutical in a nanocage and discharging the cargo inside a cell, to destroy a cancer cell, for instance; developing molecular computers that could be placed within the human body to detect disease prior to any symptoms; transporting molecules for medical reasons; or maybe even to design new types of matter.
To gain a new understanding into the behavior of matter at the nanoscale, García-Garibay’s research team developed three rotating nanomaterials called MOFs, or metal-organic frameworks, which they christened UCLA-R1, UCLA-R2 and UCLA-R3 (the “r” refers to rotor). MOFs, sometimes illustrated as crystal sponges, have pores - openings capable of storing gases, or in this case, liquid.
Analyzing the motion of the rotors allowed the team to detect the role played by a fluid’s viscosity at the nanoscale. With UCLA-R1 and UCLA-R2 the molecular rotors take up very minimal space and obstruct one another’s motion. But in the case of UCLA-R3, there was no slowing down of the rotors within the nanocage except molecules of liquid.
García-Garibay’s research team measured how quick molecules rotated in the crystals. Quadrillions of molecules can be found in each crystal rotating within a nanocage, and the chemists are aware of the position of each molecule.
UCLA-R3 was designed with large molecular rotors that travel under the pressure of the viscous forces exerted by 10 liquid molecules trapped in their nanoscale environment.
It is very common when you have a group of rotating molecules that the rotors are hindered by something within the structure with which they interact — but not in UCLA-R3. The design of UCLA-R3 was successful. We want to be able to control the viscosity to make the rotors interact with one another; we want to understand the viscosity and the thermal energy to design molecules that display particular actions. We want to control the interactions among molecules so they can interact with one another and with external electric fields.
Miguel García-Garibay, UCLA
Over the last decade, García-Garibay’s team has been working on motion in crystals and designing molecular motors in crystals. “I can get a precise picture of the molecules in the crystals, the precise arrangement of atoms, with no uncertainty,” García-Garibay said. “This provides a large level of control, which enables us to learn the different principles governing molecular functions at the nanoscale.”
García-Garibay wants to be able to develop crystals that exploit the properties of light, and whose applications could include progress in optical computing, communications technology, sensing and the field of photonics. These applications take advantage of the properties of light; light can have sufficient energy to break and create bonds in molecules.
“If we are able to convert light, which is electromagnetic energy, into motion, or convert motion into electrical energy, then we have the potential to make molecular devices much smaller,” he said. “There will be many, many possibilities for what we can do with molecular machines. We don’t yet fully understand what the potential of molecular machinery is, but there are many applications that can be developed once we develop a deep understanding of how motion takes place in solids.”
Co-authors are lead author Xing Jiang, a UCLA graduate student in García-Garibay’s laboratory, who this year completed his Ph.D.; Hai-Bao Duan, a visiting scholar from China’s Nanjing Xiao Zhuang University who spent a year conducting research in García-Garibay’s laboratory; and Saeed Khan, a UCLA crystallographer in the department of chemistry and biochemistry.
The study was funded by the National Science Foundation.
Going forward, during his tenure as dean García-Garibay will pursue his research on molecular motion in crystals and green chemistry.