Nov 29 2016
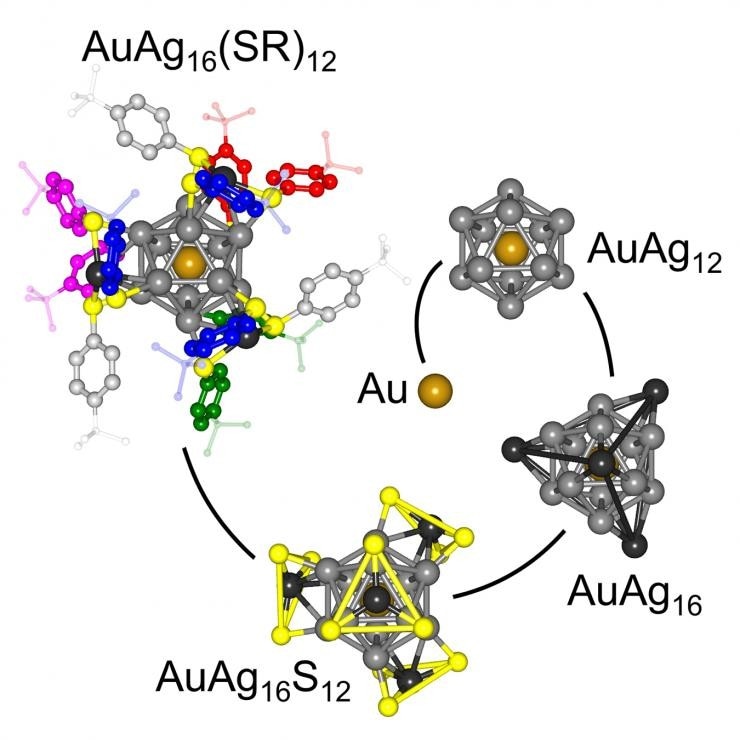 Structure evolution spiral of the AuAg16(SR)12 cluster. The buildup of the structure of the organic monolayer-capped metallic AuAg16 cluster is shown starting from the central single gold atom in the middle and its encapsulation by 12 silver atoms (gray) in an AuAg12 icosahedron. Capping of the AuAg12 core through anchoring of organic benzene-containing molecules via sulfur-containing mounts (AuAg16S12) culminates in the formation of the monolayer-capped cluster AuAg16(SR)12, with the aromatic rings of the capping monolayer exhibiting unique dimer and trimer bundled structures. (Credit: Georgia Tech / UT)
Structure evolution spiral of the AuAg16(SR)12 cluster. The buildup of the structure of the organic monolayer-capped metallic AuAg16 cluster is shown starting from the central single gold atom in the middle and its encapsulation by 12 silver atoms (gray) in an AuAg12 icosahedron. Capping of the AuAg12 core through anchoring of organic benzene-containing molecules via sulfur-containing mounts (AuAg16S12) culminates in the formation of the monolayer-capped cluster AuAg16(SR)12, with the aromatic rings of the capping monolayer exhibiting unique dimer and trimer bundled structures. (Credit: Georgia Tech / UT)
Researchers have been able to predict and verify the full structure of a monolayer-coated molecular metal nanoparticle using a combined theoretical and experimental approach.
Expanding on prior knowledge about gold nanoparticles, this methodology was tested on silver-thiolate nanoparticles, and is anticipated to be applicable to a wide range of sizes of nanoparticles made of varied elements.
The researchers from the Georgia Institute of Technology and the University of Toledo published their findings in the November 25 issue of the Science Advances journal. In the paper they describe an X-ray-determined structure that is capable of authenticating the a priori prediction, and in conjunction with first-principles theoretical analysis, supports the underlying forecasting methodology.
Metal nanoparticles capped by organic ligands have fundamental and applied significance for understanding the physical and chemical principles controlling the assembly and atomic organization in nanocrystalline materials, and to their potential usage in fields as quantum dots, sensors, nanocatalysts, biomedical imaging, nanocrystalline superlattices and plasmonics. We have been engaged in investigations of the principles underlying the structures of atomic and molecular nanoclusters, with some of our earlier predictions made over two decades ago, and thus, in some sense, the achievement demonstrated in this paper closes a circle for us, pointing the way toward future science governing atomically-precise structures at this scale.
Uzi Landman, Regent’s Professor and F.E. Callaway Chair, Georgia Institute of Technology
The research was supported by the National Science Foundation, the Air Force Office of Scientific Research, and the U.S. Department of Energy’s Office of Basic Energy Sciences. Computational research was conducted at the Georgia Tech Center for Computational Materials Science.
Since the 1990s, researchers have been studying the unique properties of metallic nanoparticles whose surfaces have been passivated using, principally, sulfur-based organic materials.
These thiol-capped structures, made up of dozens to hundreds of atoms, have unique electrical and optical properties depending on the number of metal atoms in the nanoparticle core, the metals’ chemical identities, and the type and number of capping organic ligands – all of which establish the structure of the nanoparticle.
It is a formidable theoretical goal, and an experimental challenge to predict the structure of such nanoparticles. Nanoparticles composed of various metals – copper, gold, silver, platinum, and their alloys – can be created with sizes ranging from dozens up to hundreds of atoms, being characterized by metal-specific discrete sequences of many atoms reflecting preferential stabilities and higher abundances of nanoparticles of specific sizes.
The atomic constituents in these stable nanoparticles are organized in structures that differ from the corresponding bulk atomic arrangements of the constituent metals. The plethora of varied structures formed accounts for the diversity and variability in the chemical and physical properties of such finite-size nanomaterials.
Furthermore, the metal nanoparticles are capped by sulfur-containing ligands, whose bonding to the metal constituents further complicates the structural predictions, Landman observed.
There is often something that underlies the abundance and atomic organization in such particles that is subtle and unusual. The interactions vary based on competing effects. We learned how complicated the effects of the ligand binding can be, and how to integrate this knowledge into a structure-forecasting strategy.
Uzi Landman, Regent’s Professor and F.E. Callaway Chair, Georgia Institute of Technology
Using concepts from nucleation theory and judiciously chosen trial structural motifs, partly taken from former studies, along with first-principles quantum-mechanical structure-optimization computational methods, the researchers advanced in an earlier study (published in the Journal of the American Chemical Society in 2015) a de novo predicted structure for the capped silver nanoparticle.
This prediction was mostly borne out by the subsequent experimentally established structure accomplished by a team of researchers headed by Professor Terry Bigioni from the department of Chemistry at the University of Toledo.
In the Science Advances paper, the researchers describe an experimental X-ray total structure determination and theoretical optimization and analysis of the atomic arrangement in the nanoparticle whose chemical formula is (TOA)3AuAg16 (TBBT)12, where TBBT (4-tert-butylbenzenethiol) denotes the organic thiol ligand molecules, and TOA (tetraoctylammonium) acts as a counterion.
The nanoparticle’s inner part is made up of a central gold atom surrounded by twelve silver atoms, forming a 13-atom five-fold-symmetric icosahedral metal core. The organic ligands have been predicted, and experimentally verified, to be anchored to the metal core though bonding to four more silver atoms, forming four-Ag (TBBT)3 capping mounts.
In addition to the emergence of the new mount-motif family for silver-thiolate nanoparticles, the research compares in depth the observed and predicted structural, spectral, and electronic properties of the monolayer-protected gold-atom-doped silver nanoparticle, largely verifies the de novo structure prediction as well as identifies accessible rotational isomeric ligand-shell conformations, confirms the structure forecasting methodology, and provides momentum for further theoretical and experimental developments.
Among the main points of the reported research was the growing insight concerning the probable role of the organic ligands in manipulating the structure of the nanoparticle.
If you modify the capping agent, you may modify structures, and that is a radical paradigm change. Usually, you would expect the metal nanoparticles to arrange in ways dictated by their intermetallic interactions, with only mild influence from the capping organic molecules.
Uzi Landman, Regent’s Professor and F.E. Callaway Chair, Georgia Institute of Technology
Another highlight pertains to ordering within the ligand shell, which was theoretically predicted 20 years ago in the context of investigations of capped gold nanoparticles, known as “ligand bundling.” Subsequently, such ligand orderings have been confirmed in numerous instances.
The identification of intermolecular ligand bundling in the current work, with the emergence of perennial noncovalent phenyl-ring assemblies in the form of a cyclic trimer and T-shape-like dimers, is of significance to molecular recognition, self-assembled supramolecular architecture, crystal packing, biomolecule (DNA and protein) structures, and quantum-chemistry benchmark researches.
“These findings demonstrate key principles underlying ligand-shell anchoring to the metal core, as well as unique T-like benzene-dimer and cyclic-benzene-trimer ligand bundling configurations, opening vistas for rational design of metal and alloy nanoparticles,” the authors said in the paper.
The study “provides an impetus and guidance for continued efforts toward formulation and implementation of structure prediction methodologies for such complex materials systems.”
The principles behind the ligand-shell structure also imply that the structure of bimetallic nanoparticles could be impacted by the coordination of the metal atoms in the ligand shell. For instance, if the coordination of the heteroatoms is not compatible with the ligand shell structure, then those heteroatoms will be located in the metal core. In this sense, heteroatom substitution can be used as a structural probe.
If the incompatible metal atoms are found in the ligand shell, however, then the structure of the nanoparticle will not be conserved, due to structural alterations in the ligand shell necessitated by the different heteroatom bonding needs.
Others who contributed to the research are graduate students Brian E. Conn and Aydar Atnagulov from the Department of Chemistry at the University of Toledo, Ohio, and Bokwon Yoon and Robert N. Barnett, senior research scientists in the Georgia Tech School of Physics.
This research was supported by NSF grant CBET-0955148, grant FA9550-14-1-0005 from the Air Force Office of Scientific Research, and by the Office of Basic Energy Sciences of the U.S. Department of Energy under contract FG05-86ER45234.