Nov 30 2016
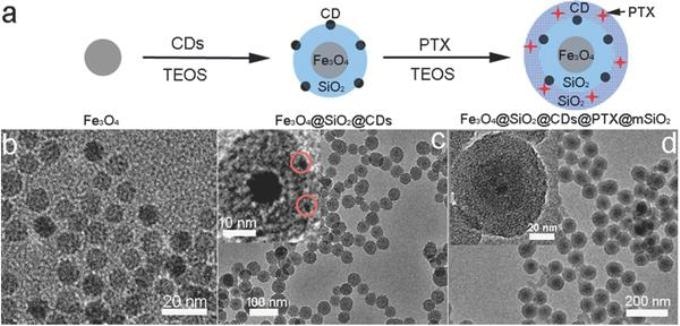 Zhang's group created this nanocarrier using a "load during assembly" approach, shown along the top. Images b, c and d are microscopic views of the nanocarriers at each major step of the assembly and loading process. (Credit - Miqin Zhang)
Zhang's group created this nanocarrier using a "load during assembly" approach, shown along the top. Images b, c and d are microscopic views of the nanocarriers at each major step of the assembly and loading process. (Credit - Miqin Zhang)
A conundrum of cancer is the ability of a tumor to use the human body as a human shield to deflect treatment. Tumors develop around normal organs and tissues, which gives doctors few options, but to damage, remove, or poison healthy parts of the body trying to fight back the cancer with surgery, radiation, or chemotherapy.
Recently in a paper published on September 27 in the journal Small, researchers at the University of Washington report a new system to encase chemotherapy drugs within miniature, synthetic “nanocarrier” packages, which could then be injected into patients and disassembled at the site of the tumor to discharge their toxic drugs.
The team, headed by UW professor of materials science and engineering Miqin Zhang, is not the first to work on nanocarriers. However, the nanocarrier package created by Zhang’s team is a hybrid of synthetic materials, which provides the nanocarrier the unique ability to carry not only drugs, but also miniature magnetic or fluorescent particles to stain the tumor and make it detectable to surgeons.
Our nanocarrier system is really a hybrid addressing two needs - drug delivery and tumor imaging. First, this nanocarrier can deliver chemotherapy drugs and release them in the tumor area, which spares healthy tissue from toxic side effects. Second, we load the nanocarrier with materials to help doctors visualize the tumor, either using a microscope or by MRI scan.
Miqin Zhang, Professor, University of Washington
Their hybrid nanocarrier builds on years of research into the different types of synthetic materials that could carry drugs for delivery into a particular part of a patient’s body.
In earlier attempts, researchers would often first try to build an empty nanocarrier using a synthetic material. Once put together, they would fill the nanocarrier with a therapeutic drug. However, this approach was inefficient, and posed a high risk of damaging the delicate drugs causing them to become ineffective.
Most chemotherapy drugs have complex structures - essentially, they’re very fragile - and they do no good if they are broken by the time they reach the tumor.
Miqin Zhang, Professor, University of Washington
Zhang’s team tried to solve this issue by designing a nanocarrier that could be assembled and loaded at the same time. Their method is similar to laying cargo within a shipping container even as the container’s floor, walls, and roof are being put together and bolted.
This “load during assembly” method also allowed Zhang’s team to add many chemical components into the structure of the nanocarrier, which could help to properly hold the cargo in place and render the tumor easy to image in clinical labs.
The core of their nanocarrier is made up of iron oxide, which not only provides structure but can also be used as an imaging agent in MRI scans. A shell of silica encases the core, and was built to efficiently stack the chemotherapy drug known as paclitaxel. They also provided space in the nanocarrier for carbon dots, miniature particles that can “stain” tissue and make it easier to observe under a microscope, helping doctors resolve the boundaries between healthy tissue and cancerous for additional treatment or surgery. The intensity of several imaging agents fades in time, but Zhang explains that their nanocarrier will provide sustained imaging for some months.
Although holding so much cargo, the completely loaded nanocarriers measure less than the thickness of a sheet of notebook paper.
The silica shell ensures that the nanocarriers are watertight. Also, they do not mess with healthy tissue, as Zhang’s team demonstrated by injecting healthy mice with empty nanocarriers or nanocarriers loaded with drug doses. Five days following the injection, they tested the vital organs in the mice for presence of toxicity and did not detect any.
This would indicate that the nanocarriers themselves do not trigger an adverse reaction in the body, and that the loaded nanocarriers are keeping their toxic cargo shielded from the body.
Miqin Zhang, Professor, University of Washington
The UW team also engineered the nanocarriers to be easily disassembled as soon as they reach a desired location. Mild heating from low-level infrared light was enough to make the nanocarriers disassemble and disgorge their cargo, which is a key feature doctors can apply to the tumor site during treatment.
Zhang’s team used mice with a type of transmissible cancer for their final test of the nanocarrier effectiveness. There was no reduction in tumor size in mice that were injected with empty nanocarriers. However, in the case of mice that were injected with nanocarriers that were loaded with paclitaxel, the tumors shrank considerably. A similar effect was noticed on human cancer cells cultured and examined in the lab.
These results show that the nanocarriers can deliver their cargo intact to the tumor site, and while we designed this nanocarrier specifically to accommodate paclitaxel, it is possible to adjust this technique for other drugs.
Miqin Zhang, Professor, University of Washington
Although there are numerous phases to pass before this technology is proven safe and effective for humans, Zhang hopes her team’s method and promising results will aid in speeding the progress.
The paper’s lead authors are Hui Wang and Kui Wang in the UW Department of Materials Science & Engineering. Co-authors are Bowei Tian in the UW Department of Applied Mathematics and Richard Revia, Qingxin Mu, Mike Jeon, Fei-Chien Chang — all in the Department of Materials Science & Engineering. The research received funding from the National Institutes of Health and the University of Washington.