Aug 7 2017
A catalyst based on laser-induced grapheme, which splits water into hydrogen on one side and oxygen on the other side, has been produced by Rice University Chemists. According to the Chemists, the cost-effective material may be a practical component in producing the hydrogen for use in future fuel cells.
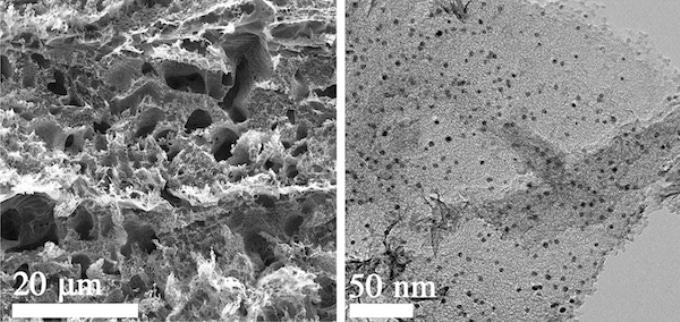 A two-sided electrocatalyst developed at Rice University splits water into hydrogen on one side and oxygen on the other. The hydrogen side seen in electron microscope images features platinum particles (the dark dots at right) evenly dispersed in laser-induced graphene (left). Courtesy of the Tour Group
A two-sided electrocatalyst developed at Rice University splits water into hydrogen on one side and oxygen on the other. The hydrogen side seen in electron microscope images features platinum particles (the dark dots at right) evenly dispersed in laser-induced graphene (left). Courtesy of the Tour Group
The Rice lab of Chemist James Tour has developed this effortlessly fabricated material, which offers an efficient and robust way to store chemical energy. Tests revealed the thin catalyst generating huge bubbles of hydrogen and oxygen on either side simultaneously.
The process has been the main theme of a paper published in the American Chemical Society’s Applied Materials and Interfaces.
Hydrogen is currently made by converting natural gas to a mixture of carbon dioxide and hydrogen gas. So for every two hydrogen molecules, a molecule of carbon dioxide is formed, making this traditional process a greenhouse-gas emitter. But if one splits water into hydrogen and oxygen, using a catalytic system and electricity generated from wind or solar energy, then the hydrogen afforded is entirely renewable. Once used in a fuel cell, it reverts back to water with no other emissions. And fuel cells are often twice as efficient as internal combustion engines, further saving energy.
James Tour, Chemist, Rice University
The catalyst is another use for flexible laser-induced graphene (LIG), which was introduced by Rice in 2014. LIG is developed by treating the surface of a sheet of polyimide, a cost-effective plastic, with a laser. Instead of being a flat sheet of hexagonal carbon atoms, LIG is considered to be a foam of graphene sheets with one edge fixed to the underlying surface and chemically active edges that are exposed to the air.
Turning LIG into a water splitter involves a few more steps as it is inert by itself. The lab first impregnated the side of the plastic intended to pull hydrogen from water with platinum particles; this was followed by using a laser to heat the surface and produce LIG. The Rice material uses just a quarter of the platinum detected in commercial catalysts, explained Jibo Zhang, a Rice Graduate Student and Lead Author of the paper.
The other side, for oxygen evolution, was initially turned into LIG and then improved with iron and nickel via electrochemical deposition. Both sides revealed strong performance over 1,000 cycles and low onset potentials (the voltage required for starting a reaction).
The lab brought about another variation: producing the polyimide into an LIG catalyst with phosphorus and cobalt that would replace either the platinum or nickel-iron sides in order to generate oxygen or hydrogen. The cost-effective material benefits by eradicating expensive noble metals, and thus sacrifices some effectiveness in hydrogen generation, Tour said.
The catalyst, when configured with nickel-iron for oxygen and cobalt-phosphorus for hydrogen evolution, delivered a current density of 10 milliamps per square centimeter at 1.66 volts. This could be increased to 400 milliamps per square centimeter at 1.9 volts without the material being degraded. The rate of the chemical reaction is governed by the current density.
According to Tour, LIG offers water-splitting performance that is comparable and often considered to be better than many current systems, with an advantage in its inherent separator between hydrogen and oxygen products. He noted it may find immense value as a way for chemically storing energy from wind power or remote solar plants that would otherwise be lost in transmission.
The material could also serve as the basis for effective electrocatalysis platforms for carbon dioxide or oxygen reduction, he stated.
Co-authors include Graduate Students Chenhao Zhang, Huilong Fei, Yilun Li and Junwei Sha. Sha is also a Student at Tianjin University and the Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, China. Tour is the T.T. and W.F. Chao Chair in Chemistry and also a Professor of Computer Science and of Materials Science and Nanoengineering at Rice.
The Air Force Office of Scientific Research, the National Science Foundation-funded and Rice-based Nanotechnology-Enabled Water Treatment Engineering Research Center and the Chinese Scholarship Council supported the research.