Oct 20 2017
A new way to increase the sensitivity of detecting volatile compounds, especially chlorine, using metallic nanoparticles, has been developed by researchers from the Faculties of Chemistry and of Materials Science of Lomonosov Moscow State University. The work features in the Talanta journal.
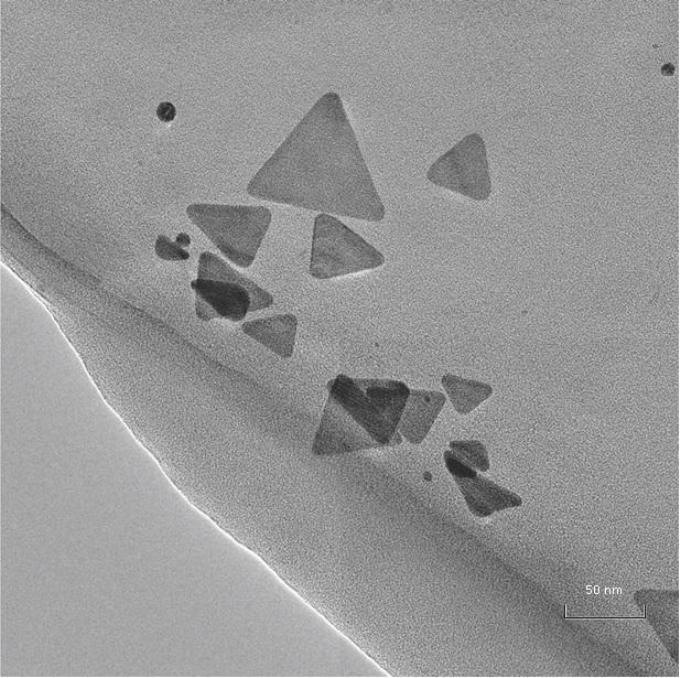 This is an image of TEM micrograph of aqueous solution of silver triangular nanoplates. (CREDIT: Aleksei Furletov)
This is an image of TEM micrograph of aqueous solution of silver triangular nanoplates. (CREDIT: Aleksei Furletov)
Metallic nanoparticles, specifically the nanoparticles of silver and gold, are extensively used in analytical chemistry. Amongst their uses is developing optical sensors based on the surface plasmonic resonance (the phenomenon of surface plasmon excitation by light) on solid supports and in colloidal solutions. Modern optical sensors have significant advantages such as ease of detecting an analytic signal, high sensitivity and adjustability of the laboratory and optical analysis parameters. However, when it comes to selectivity, these devices have specific limitations.
It occurs because of aggregative instability of nanoparticles (the particles stick together when they collide) which begins during high ionic strength (high intensity of the electric field produced by ions). The ion layer produced on the surface of particles is known as the double electric layer and is characterized by an electrokinetic potential, also referred to as the zeta potential. The electrostatic stabilization of nanoparticles does not take place due to a decrease in the zeta potential.
The problem can be resolved if the nanoparticles are fixed to solid supports; scientists then obtain micro- or nanosensors based on solid particles. Many matrix materials are not available for these sensors, and the process of attaching the nanoparticles to supports is a complicated one, thus the researchers started working on a problem of adjusting the surface of sensor matrices. For this, they suggested separating the nanoparticles from chemical compounds and ions while retaining their sensitivity.
The Russian chemists developed a technique capable of integrating optical detection using paper test strips with triangular silver nanoparticles spread all over them, and dynamic gas extraction, which refers to the extraction of a compound from a dry mixture or a solution by means of liquefied gases. Perspectives of this technique were exposed by detecting chlorine. Chlorine is frequently used for purifying water, as it destroys the outer shell of viruses and bacteria. However, the problem of determining the chlorine concentration in water continues to be relevant, since the current techniques are not adequately sensitive.
"The technique developed allows to determine small amounts of gaseous chlorine in the presence of large concentrations of foreign compounds without any sample preparation. This approach can be applied to other analytical systems based on metal nanoparticles, which opens up broad opportunities for the further development of this area of chemical analysis. "
Aleksei Furletov, student of the Department of Analytic Chemistry, Faculty of Chemistry, Lomonosov Moscow State University