Jan 11 2019
Benzene—a common hydrocarbon—has been used by researchers for the first time to produce a new form of molecular nanotube, which could pave the way to advanced nanocarbon-based semiconductor applications.
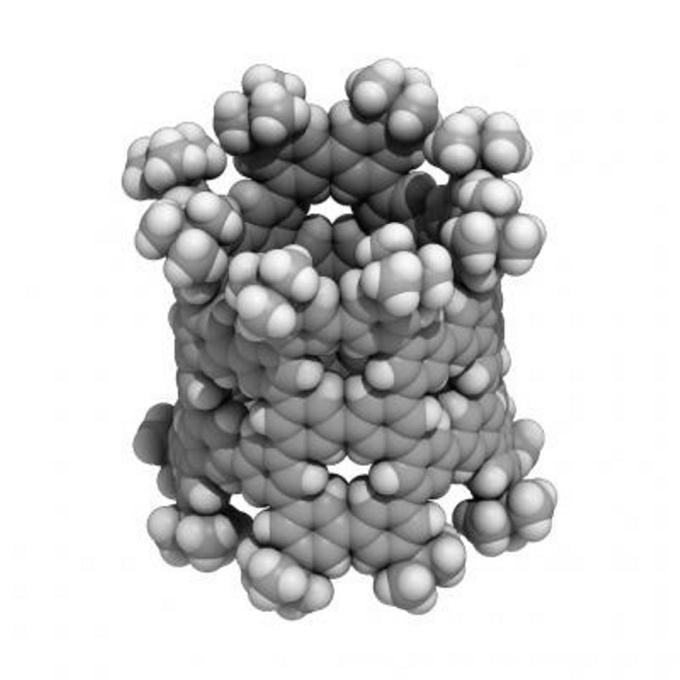 A nanometer-sized pNT cylinder made of 40 benzenes. The cylinder is tens of thousands of times thinner than a human hair. (Image credit: (c)2018 Hiroyuki Isobe)
A nanometer-sized pNT cylinder made of 40 benzenes. The cylinder is tens of thousands of times thinner than a human hair. (Image credit: (c)2018 Hiroyuki Isobe)
At the Department of Chemistry, researchers have been working strenuously in their newly renovated laboratory at The University of Tokyo’s Graduate School of Science. The smart layout and the pristine environment provide the researchers abundant opportunities for interesting experiments. An appreciation for “beautiful” molecular structures led Professor Hiroyuki Isobe and coworkers to develop something that is both beautiful and a first in the field of chemistry.
The team’s phenine nanotube (pNT), with its pleasing simplicity and symmetry, is extremely aesthetic and is a stark contrast to its intricate means of coming into being. Considerable difficulty and challenges are involved in the chemical synthesis of nanotubes, even more so if one wants to subtly regulate the target structures to offer special functions and properties.
Although standard carbon nanotubes are known for their defect-free and perfect graphite structures, they tend to differ extensively in terms of diameter and length. Isobe and his group wanted a single type of nanotube, a new form that has controlled defects inside its nanometer-sized cylindrical structure enabling more molecules to add functions and properties.
The researchers’ innovative synthesis process begins with benzene, which is a hexagonal ring of six carbon atoms. By using reactions and by integrating six of these benzenes, the researchers created a larger hexagonal ring known as a cyclo-meta-phenylene (CMP). Next, platinum atoms were used which enabled four CMPs to create an open-ended cube. The removal of the platinum causes the cube to spring into a dense circle and this is furnished using bridging molecules on either ends thus enabling the tube shape.
While it appears to be highly complicated, this intricate process is rather incredible and effectively bonds the benzenes in the right way over 90% of the time. Here, the molecule’s symmetry also plays a role and this molecule eases the process to assemble as many as 40 benzenes. These benzenes, also known as phenines, are utilized as panels to form a cylinder of nanometer-size. The outcome is an innovative nanotube structure that has deliberate periodic defects. Hypothetical analyses revealed that these periodic defects imbue the nanotube with semiconductor characters.
A crystal of pNT is also interesting: The pNT molecules are aligned and packed in a lattice rich with pores and voids. These nanopores can encapsulate various substances which imbue the pNT crystal with properties useful in electronic applications. One molecule we successfully embedded into pNT was a large carbon molecule called fullerene (C70). A team lead by Kroto/Curl/Smalley discovered fullerenes in 1985. It is said that Sir Harold Kroto fell in love with the beautiful molecule. We feel the same way about pNT. We were shocked to see the molecular structure from crystallographic analysis. A perfect cylindrical structure with fourfold symmetry emerges from our chemical synthesis. After a few decades since the discovery, this beautiful molecule, fullerene, has found various utilities and applications. We hope that the beauty of our molecule is also pointing to unique properties and useful functions waiting to be discovered.
Hiroyuki Isobe, Professor, Department of Chemistry, The University of Tokyo.