Feb 19 2019
A revolutionary nanoscience discovery has been made by a research team that included Professor Uri Banin, founder of the Hebrew University of Jerusalem’s Center for Nanoscience and Nanotechnology and his colleagues Professors Tobias Hanrath and Richard Robinson at Cornell University.
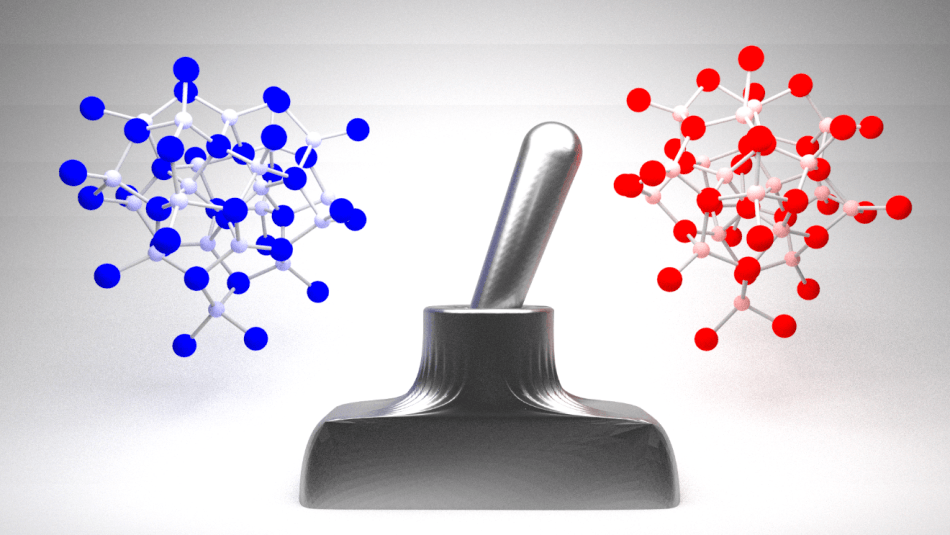 Magic-size nanoclusters behaves like a switch, changing from one form to another in a single step. (Image credit: Douglas Nevers/Cornell University)
Magic-size nanoclusters behaves like a switch, changing from one form to another in a single step. (Image credit: Douglas Nevers/Cornell University)
In their latest paper titled, “Chemically Reversible Isomerization of Inorganic Clusters” featured in Science, the authors demonstrated that the missing link is a “magic-size nanocluster” that connects the divide between the way matter rearranges itself in huge and solid bulk matter phase transitions and in small-scale molecular isomerization.
Isomerization, in which a molecule is converted into another molecule with the same atoms but in a different arrangement, is quite common in nature. This phenomenon is usually triggered upon adding energy, just like how light promotes a molecule in the retina to switch, allowing humans to see; or the way olive oil, when heated to high temperatures, isomerizes into the unhealthy form called a trans-fat. Although graphite and other similar bulk materials can also change phases—into diamonds, for instance—they need plenty of energy and the change takes place more slowly, with the change gradually spreading over the molecule.
Researchers have long been seeking to link between the two worlds—tiny organic materials that have the ability to flip back and forth in a coherent way between two states, and huge materials that alter more gradually. This continued to be the mysterious missing link in nanoscientists’ quest for mapping and inferring the crossover from molecular isomerization to phase transitions. To find out this bridge, the researchers have to find at what size nanocrystals will alter their internal structure in one, rapid step, similar to how molecules do at the time of isomerization. Robinson and Banin discovered that magic number on an unexpected flight from Ithaca to Jerusalem.
Three years before, Robinson was on sabbatical at Banin’s nanoscience laboratory at Hebrew University. While Robinson was in Jerusalem, he requested a graduate student from back home to send him some nanoparticles of a certain size.
“When they got to me, I measured them with the spectrometer and I said, ‘Wait, you sent me the smaller particles instead of the bigger ones.’ And he said, ‘No, I sent you the bigger ones,” recalled Robinson. “We realized they must have changed while they were in flight. And that unleashed a cascade of questions and experiments that led us to this new finding.”
Robinson, Banin, and Hanrath inferred that the particles had changed during their flight from Ithaca to Jerusalem.
On the flight there must have been moisture in the cargo bin and the samples switched their phase.
Uri Banin, Professor and Founder, Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem
The joint Cornell and Hebrew University team started to analyze this transition in tiny cluster molecules, particularly “magic size nanoclusters.” These nanoclusters contain just 57 atoms—rendering them bigger than the regular molecules but still relatively smaller compared to bulk materials, such as diamonds or graphite. The researchers showed that the change in these clusters—as they transitioned from one structure, or phase, to another—occurred in one, rapid step, as is the case for isomerization in tiny molecules. In this manner, the researchers covered the elusive missing link between molecular isomerization and bulk phase transitions.
Although more research is required, these particles can possibly be used as sensors or as switches in computing in the future, stated Banin. In addition, this latest breakthrough could have applications pertaining to quantum computing or even as a seed for creating bigger nanoparticles.
One hundred years ago, Albert Einstein could not have predicted that his Theory of Relativity would be the basis for GPS systems and the Waze app which we’ve come to rely on for navigation. Nanoclusters are chemicals that can be used to create other larger materials. Being able to manipulate their precise changeover from one state to another could have many significant applications down the road.
Uri Banin, Professor and Founder, Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem