Apr 1 2019
Scientists have engineered two types of nano-sized building blocks that can automatically connect into cubes and scramble back into separate components based on the temperature of their environment. This achievement is one step closer to chemical systems that more convincingly mimic life.
 Professor Shuichi Hiraoka's research group has designed two types of nanocubes whose building blocks, hexaphenylbenzene derivative molecules (represented as red and blue), can automatically combine and scramble repeatedly based on the temperature of their environment. (Image credit: Zhan et al. 2019 DOI: 10.1038/s41467-019-09495-1)
Professor Shuichi Hiraoka's research group has designed two types of nanocubes whose building blocks, hexaphenylbenzene derivative molecules (represented as red and blue), can automatically combine and scramble repeatedly based on the temperature of their environment. (Image credit: Zhan et al. 2019 DOI: 10.1038/s41467-019-09495-1)
Imagine mixing two liquids together, like ink and water. They will automatically do the simple chemical process of dispersing until they are perfectly mixed. However, there’s no chemical process to automatically separate water and ink–you need to boil the water into a gas to separate it from the ink.
Shuichi Hiraoka, Professor, Department of Basic Science, University of Tokyo.
The new building blocks developed by Hiraoka's team can scramble or connect themselves automatically because of the varying temperature stabilities of the cubes that they form.
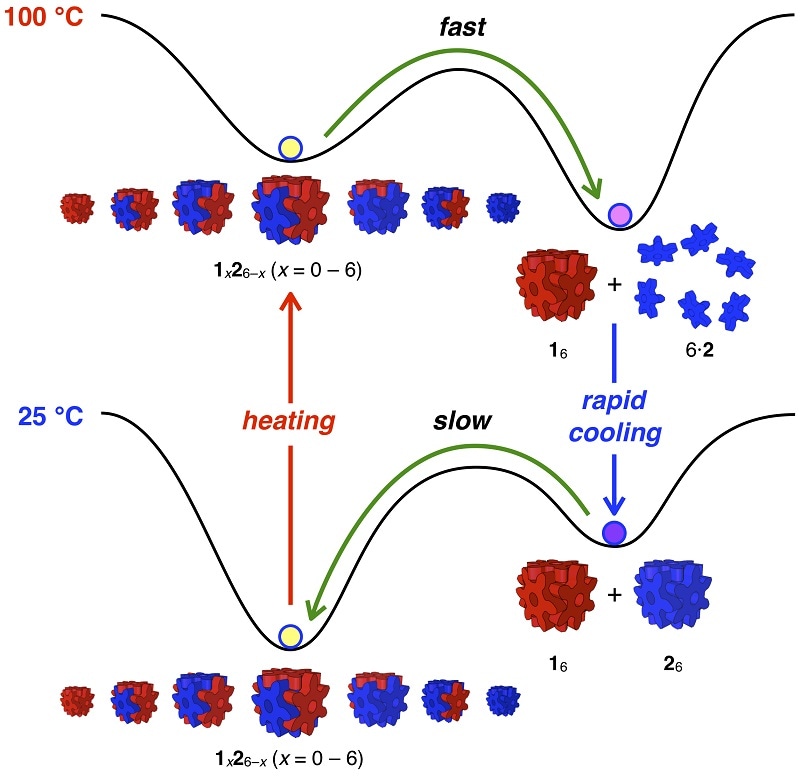
The two types of building blocks are practically identical and resemble snowflakes. Scientists color them blue or red in schematic diagrams. A cube made from red blocks is stable below 130 °C (266 °F), while a cube made from blue blocks is stable only below 65 °C (149 °F).
When kept separately, the building blocks connect into completely red or completely blue uniform cubes.
When the two types of cubes are blended together at room temperature (25 °C, 77 °F), the red and blue building blocks automatically re-join into the most stable type of cubes, which are made of any mixture of three red and three blue blocks. This mixture of red-blue cubes is similar to properly-mixed ink and water.
When scientists aim to scramble the red-blue cubes to reform completely red and completely blue cubes, they perform a simple two-step temperature change. This ability signifies a chemical feat not possible with a blend of ink and water.
First, the mixture is heated up to boiling (100 °C, 212 °F). The blue cubes become unstable and float off as separate molecules, while the red building blocks recombine into completely red cubes.
Then, scientists quickly cool the mixture (0 °C, 32 °F) so that the red cubes remain together while the blue building blocks automatically assemble into completely blue cubes.
Scientists can also stop a mixture of completely red and completely blue cubes from scrambling into the more stable red-blue blended cubes by filling the cubes with some substance, for instance, other molecules such as hydrocarbons. The guest molecules successfully lock the cubes closed from the inside so that the building blocks will not be able to scramble into other, more stable cube groupings.
In the future, we plan to develop an even more complicated chemical system based on molecular cubes, using the unique self-assembly ability and a variety of energy sources beyond just heat.
Shuichi Hiraoka, Professor, Department of Basic Science, University of Tokyo.
The nanocubes in the present study have the same standard structure as the design stated in October 2018.