Jul 18 2019
A single oxygen atom can never be easily pinned down, and it is totally impossible to then manipulate electrons in that single atom to modify its charge. However, an international team of researchers led by Osaka University has reported this achievement for the first time.
 (Image credit: Osaka University)
(Image credit: Osaka University)
In collaboration with colleagues from Slovakia and the United Kingdom, graduate student Yuuki Adachi from Osaka University’s Department of Applied Physics has recently reported this study in ACS Nano.
Oxygen, which is one of the most abundant elements found on Earth, is often found in its diatomic form—O2. It is highly reactive and does not exist in a gaseous state for a long time. The least reactive form of oxygen, or its ground state, is termed as triplet oxygen since it has three probable arrangements of electron spins.
By contrast, singlet oxygen has only one possible spin arrangement. Therefore, it is more reactive and has a vital role to play in a wide range of chemical reactions, from green fuel production to photodynamic cancer treatments. Hence it is not surprising that considerable attention is paid toward controlling the formation and activation of molecular oxygen.
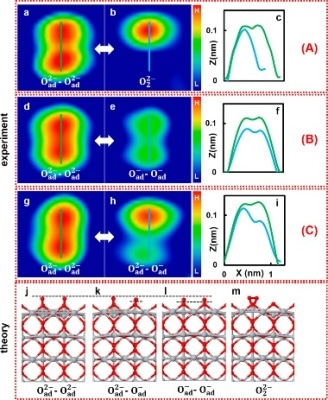
We used Kelvin probe force spectroscopy to examine the charge states of oxygen atoms attached to a titanium dioxide rutile surface, and to then manipulate the charge through the transfer of individual electrons to and from pairs of oxygen atoms.
Yuuki Adachi, Graduate Student, Department of Applied Physics, Osaka University
Adachi added, “We identified three different charge states amongst the pairs: O−/O−, O2−/O2−, and O−/O2−. Depending on the applied voltage and where we positioned the tip of the probe relative to the atoms, we could then reversibly switch the charge between the O− and O2− states.”
Then, the researchers demonstrated that the same method could be used to induce controlled, reversible bond formation between two neighboring oxygen atoms, thereby forming molecular oxygen (O2).
Fascinatingly, it was also discovered that it is possible to remotely control the charge state by locating the tip elsewhere on the rutile surface. The transfer of electrons to the oxygen atoms was realized using surface polarons, a process in which electrons can move through a crystal lattice.
This level of control over the charge state of oxygen atoms has not previously been possible. Our work provides a novel method to examine transition-metal-oxide-based catalytic reactions, and can likely be applied to other atoms, and perhaps other surfaces, where controlled chemical reactions initiated by charge manipulation are performed.
Yan Jun Li, Study Corresponding Author and Associate Professor, Osaka University