Aug 7 2019
Researchers at the University of Leeds have developed a new type of gold which has a thickness of just two atoms—the thinnest unsupported gold ever produced.
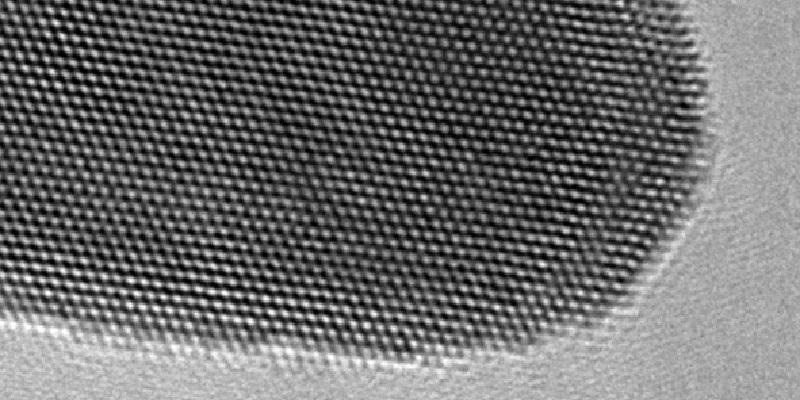 This electron microscope picture shows the gold atoms’ lattice structure. (Image credit: University of Leeds)
This electron microscope picture shows the gold atoms’ lattice structure. (Image credit: University of Leeds)
The scientists measured the gold’s thickness to be 0.47 nm—one million times thinner than a human fingernail.
The material is considered as 2D since it is made up of just two layers of atoms placed on top of one another. All atoms are surface atoms, there are no ‘bulk’ atoms buried under the surface.
This ultra-thin gold could find large-scale applications in the medical device and electronics industries, and even as a catalyst to expedite chemical reactions in a variety of industrial processes.
Laboratory tests demonstrate that the ultra-thin gold is 10 times more effective as a catalytic substrate than the larger gold nanoparticles presently employed in industry.
Landmark Achievement
Researchers think the new material could also lay the foundation of artificial enzymes for prospective application in water purification systems and in quick, point-of-care medical diagnostic tests.
The statement that the ultra-thin metal had been successfully produced was made in Advanced Science.
This work amounts to a landmark achievement. Not only does it open up the possibility that gold can be used more efficiently in existing technologies, it is providing a route which would allow material scientists to develop other 2D metals. This method could innovate nanomaterial manufacturing.
Dr Sunjie Ye, Study Lead Author, Leeds’ Molecular and Nanoscale Physics Group and the Leeds Institute of Medical Research
The team of scientists is aiming to work with industry on methods of scaling up the process.
The production of the gold nanosheet occurs in an aqueous solution and begins with chloroauric acid, an inorganic substance that consists of gold. It is reduced to its metallic form in the presence of a “confinement agent”—a chemical that promotes the gold to develop as a sheet with a thickness of just two atoms.
The gold’s nanoscale dimensions make it appear green in the water, and due to its frond-like shape, the investigators call it as gold nanoseaweed. Pictures captured by an electron microscope show the way the gold atoms have developed into a highly organized lattice structure.
Professor Stephen Evans is the head of Leeds’ Molecular and Nanoscale Research Group and directs the study. He stated that the significant benefits that could be obtained from using ultra-thin gold sheets were down to their high surface-area-to-volume ratio.
Gold is a highly effective catalyst. Because the nanosheets are so thin, just about every gold atom plays a part in the catalysis. It means the process is highly efficient. Our data suggest that industry could get the same effect from using a smaller amount of gold, and this has economic advantages when you are talking about a precious metal.
Professor Stephen Evans, Head, Leeds’ Molecular and Nanoscale Research Group
In addition, the flakes are flexible, implying that they could lay the foundation of electronic components for pliable screens, transparent conducting displays, and electronic inks.
Professor Evans believes that there will certainly be comparisons made between the 2D gold and the first-ever produced 2D material—graphene, which was created at the University of Manchester in 2004.
He said: “The translation of any new material into working products can take a long time and you can't force it to do everything you might like to. With graphene, people have thought that it could be good for electronics or for transparent coatings, or as carbon nanotubes that could make an elevator to take us into space because of its super strength.”
I think with 2D gold we have got some very definite ideas about where it could be used, particularly in catalytic reactions and enzymatic reactions. We know it will be more effective than existing technologies—so we have something that we believe people will be interested in developing with us.
Professor Stephen Evans, Head, Leeds’ Molecular and Nanoscale Research Group
The University of Leeds is renowned for its exploration in material science. It is in charge of the Bragg Centre, where academics from various fields team up. The center has access to several best facilities in the world.