Aug 12 2019
At the human level, regulating temperature is a simple concept. Turtles expose themselves to the sun to keep warm. In order to cool a pie fresh from the oven, it is placed on a countertop at room temperature.
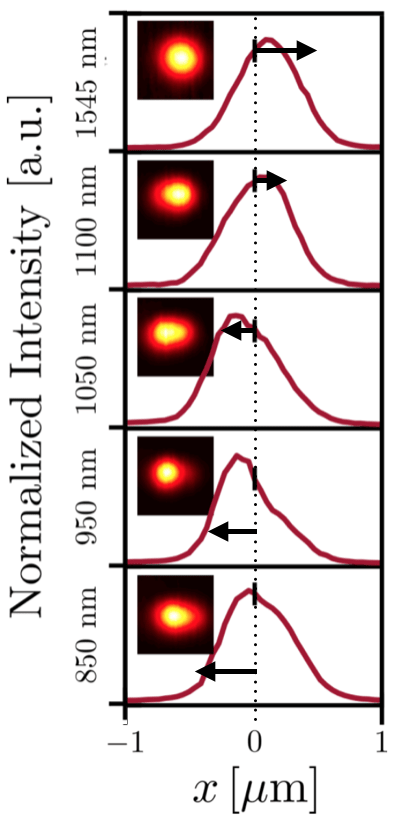 This figure shows evidence that the two nanorods were heated to different temperatures. The researchers collected data on how the heated nanorods and surrounding glycerol scattered photons from a beam of green light. The five graphs show the intensity of that scattered light at five different wavelengths, and insets show images of the scattered light. Arrows indicate that peak intensity shifts at different wavelengths, an indirect sign that the nanorods were heated to different temperatures. (Image credit: Bhattacharjee et al., ACS Nano, 2019)
This figure shows evidence that the two nanorods were heated to different temperatures. The researchers collected data on how the heated nanorods and surrounding glycerol scattered photons from a beam of green light. The five graphs show the intensity of that scattered light at five different wavelengths, and insets show images of the scattered light. Arrows indicate that peak intensity shifts at different wavelengths, an indirect sign that the nanorods were heated to different temperatures. (Image credit: Bhattacharjee et al., ACS Nano, 2019)
At the nanoscale—at distances below 1/100th the width of the thinnest strand of human hair—regulating temperature is much more complicated. Nanoscale distances are so small that objects become thermally coupled easily: If one object heats up to a particular temperature, its adjacent object also gets heated up.
When a beam of light is used as the heat source, there is another problem: Due to heat diffusion, materials in the beam path heat up to almost the same temperature, rendering it difficult to control the thermal profiles of objects within the beam.
It has not been possible for researchers to use only light to actively shape and control thermal landscapes at the nanoscale. At least, not so far.
In a paper published online on July 30th, 2019, by the ACS Nano journal, a group of scientists describes that they have developed and tested an experimental system that employs a near-infrared laser to actively heat two gold nanorod antennae—metal rods designed and constructed at the nanoscale—to different temperatures. The nanorods are too close together that they are coupled thermally as well as electromagnetically.
However, the group headed by scientists from the University of Washington, Rice University, and Temple University measured temperature variations between the rods as high as 20 ○C. By just varying the wavelength of the laser, they could also differentiate which nanorod was cooler and which was warmer, although the rods were composed of the same material.
If you put two similar objects next to each other on a table, ordinarily you would expect them to be at the same temperature. The same is true at the nanoscale. Here, we can expose two coupled objects of the same material composition to the same beam, and one of those objects will be warmer than the other.
David Masiello, Study Lead Corresponding Author and Professor of Chemistry, University of Washington
Masiello is also the faculty member in both the Molecular & Engineering Sciences Institute and the Institute for Nano-Engineered Systems.
Masiello’s group carried out theoretical modeling to develop this system. He collaborated with co-corresponding authors Stephan Link, a professor of both chemistry and electrical and computer engineering at Rice University, and Katherine Willets, an associate professor of chemistry at Temple University, to develop and test it.
Their system comprised of two nanorods composed of gold—one which is 150 nm long and the other which is 250 nm long, or around100 times thinner than the thinnest strand of human hair. The scientists kept the nanorods close to each other, end to end on a glass slide surrounded by glycerol.
They preferred gold for a particular reason. In response to sources of energy such as a near-infrared laser, electrons within gold can “oscillate” easily. These electronic oscillations, or surface plasmon resonances, effectively turn light into heat.
While both nanorods were composed of gold, their varying size-dependent plasmonic polarization indicated that they had various patterns of electron oscillations. Masiello’s group estimated that if the nanorod plasmons oscillated with either the same or contrary phases, they could reach different temperatures—counteracting the effects of thermal diffusion.
Link’s and Willets’ teams developed the experimental system and tested it by illuminating a near-infrared laser on the nanorods. They investigated the beam’s effect at two wavelengths—one for oscillating the nanorod plasmons with the same phase, another for the opposite phase.
The group could not directly measure the temperature of each nanorod at the nanoscale. Instead, they gathered data on how the heated nanorods and surrounding glycerol dispersed photons from a separate beam of green light. Masiello’s group analyzed those data and found out that the nanorods refracted photons from the green beam differently because of nanoscale variations in temperature between the nanorods.
This indirect measurement indicated that the nanorods had been heated to different temperatures, even though they were exposed to the same near-infrared beam and were close enough to be thermally coupled.
Claire West, Study Co-Lead author and Doctoral Candidate, Department of Chemistry, University of Washington
The group also identified that, by varying the wavelength of near-infrared light, they could vary which nanorod—short or long—heated up more. The laser could typically serve as a tunable “switch,” altering the wavelength to change which nanorod was hotter. The temperature variations between the nanorods also changed depending on the distance between them but reached as high as 20 ○C beyond room temperature.
The group’s findings have a variety of applications on the basis of controlling the temperature at the nanoscale. For instance, researchers could design materials that photo-thermally control chemical reactions with nanoscale precision or temperature-triggered microfluidic channels for filtering tiny biological molecules.
The scientists are working to develop and test more complex systems, like clusters and arrays of nanorods. These need more complex modeling and calculations. However, with the advancement so far, Masiello is hopeful that this exclusive collaboration between theoretical and experimental research teams will keep making progress.
“It was a team effort, and the results were years in the making, but it worked,” stated Masiello.
West’s co-lead authors on the paper include Ujjal Bhattacharjee, a former researcher at Rice University, presently at the Indian Institute of Engineering Science and Technology, Shibpur, and Seyyed Ali Hosseini Jebeli, a researcher at Rich University.
Co-authors of the study include Harrison Goldwyn and Elliot Beutler, both doctoral students in the UW Department of Chemistry; Xiang-Tian Kong and Zhongwei Hu, both research associates in the UW Department of Chemistry; and Wei-Shun Chang, a former research scientist at Rice, presently an assistant professor of chemistry and biochemistry at the University of Massachusetts Dartmouth.
The study was funded by the National Science Foundation, the Robert A. Welch Foundation, and the University of Washington.