Aug 30 2019
Carbon nanoparticles serve as a potential tool in biomedical applications. For instance, they are used in the targeted delivery of biologically active compounds into the body cells.
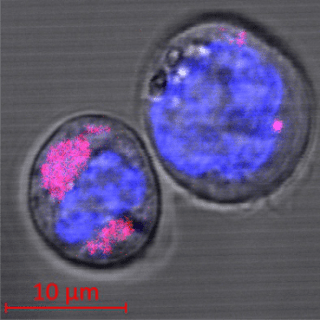 Two CD34+ stem cells containing carbon nanoparticles (colored magenta); the cell nuclei can be seen in blue. The researchers found that the nanoparticles are encapsulated in the cell lysosomes. (Image credit: HHU/Stefan Fasbender)
Two CD34+ stem cells containing carbon nanoparticles (colored magenta); the cell nuclei can be seen in blue. The researchers found that the nanoparticles are encapsulated in the cell lysosomes. (Image credit: HHU/Stefan Fasbender)
Now, a research team from the Physics, Medicine and Chemistry departments at Heinrich Heine University Düsseldorf (HHU) has studied the nature of these particles to determine whether they are potentially harmful to organisms and how cells are able to deal with them after they have been integrated. The results of the interdisciplinary study have been recently reported in the Scientific Reports journal.
Nanoparticles measure smaller than 5 nm—a nanometer is one-millionth of a millimeter—which is approximately equal to the size of macromolecules. Small particles like these are easily absorbed in the body cells.
This feature has two aspects. First, nanoparticles are made into excellent vehicles for targeted delivery of a wide range of substances or compounds (attached to the nanoparticles) into normal and diseased cells.
However, nanoparticles can also pose health risks, for instance, in relation to the particulate matter. Combustion processes are one of the methods where particulate matter is generated, and some of that can be categorized as nanoparticles.
These particles are small enough to cross the blood-air barrier and enter into the body: Lungs contain the bronchial mucosa, which does not filter out the particles, thus allowing them to penetrate the pulmonary alveoli and eventually end up in the bloodstream.
In collaboration with work groups from the Chemistry department, HHU scientists from the Department of Haematology, Oncology, and Clinical Immunology working under Professor Dr Rainer Haas and from the Institute of Experimental Condensed Matter Physics working under Professor Dr Thomas Heinzel have now discovered what exactly takes place when such nanoparticles are absorbed by the body cells.
The scientists utilized nanoparticles fabricated from graphene—a unique form of carbon containing two-dimensional (2D) layers of hexagonal carbon rings. They subsequently added these graphene-based nanoparticles to unique hematopoietic stem cells known as CD34+ stem cells.
These types of cells tend to divide throughout their lifetime, and hence they are specifically responsive to damaging environmental effects. It is believed that compared to the other and more robust cell types, the stem cells are more likely to be damaged by nanoparticles.
The interdisciplinary team of scientists located in Düsseldorf demonstrated that these carbon nanoparticles enter into the cells, where they are enclosed in lysosomes which are special organelles. These lysosomes act as a kind of waste removal unit for the body, where foreign bodies build up and are usually disintegrated by means of enzymes. Conversely, the investigators did not find any such process throughout the period of the experiments, which went on for many days.
When the researchers compared the stem cells’ active genes (“gene expression”) with and without the inclusion of nanoparticles, they observed changes in only one of a total of 20,800 recorded expressions; slight effects were measured in an additional 1,171 gene expressions.
Encapsulation of the nanoparticles in the lysosomes ensures that these particles are stored securely at least for a few days—for the duration of our experiments—and cannot damage the cell. This means the cell remains viable without any major change in gene expression.
Dr Thomas Heinzel, Professor, Institute of Experimental Condensed Matter Physics, HHU
This perception is significant if nanoparticles need to be used for transporting drugs into the cell. However, the experimental framework employed in this case does not permit any long-term statements to be made with regards to the increased possibility of cell mutation leading to cancer.
The study was performed as a close association between HHU’s Faculty of Mathematics and Natural Sciences and Faculty of Medicine and University Hospital Düsseldorf. The doctoral scholarship of first author Stefan Fasbender was funded by Düsseldorf School of Oncology (led by Professor Dr Sebastian Wesselborg).
The proximity of the Hospital and the University and their close links in terms of content provides HHU with a particularly fruitful environment for translational research, where insights and expertise from basic research are combined with aspects relevant to treatment.
Dr Rainer Haas, Professor, Department of Haematology, Oncology, and Clinical Immunology, HHU