Nov 14 2019
In a new study, engineers from the University of Illinois combined atomic-scale experimentation with computer modeling to quantify the energy required to bend multilayer graphene.
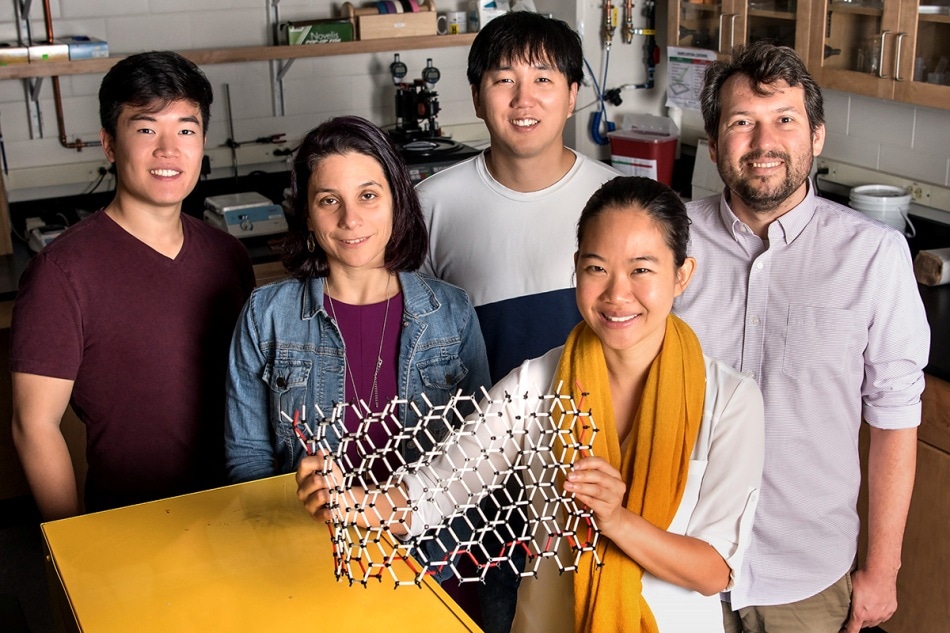 Graduate student Edmund Han, left, professor Elif Ertekin, graduate student Jaehyung Yu, professor Pinshane Y. Huang, front, and professor Arend M. van der Zande have determined how much energy it takes to bend multilayer graphene—a question that has long eluded scientists. Image Credit: Stephanie Adams.
Graduate student Edmund Han, left, professor Elif Ertekin, graduate student Jaehyung Yu, professor Pinshane Y. Huang, front, and professor Arend M. van der Zande have determined how much energy it takes to bend multilayer graphene—a question that has long eluded scientists. Image Credit: Stephanie Adams.
This question has eluded researchers from the time graphene was first isolated. The study outcomes have been published in the Nature Materials journal.
Graphene is formed of a single layer of carbon atoms arranged in a lattice, and is the strongest material in the world. According to the researchers, it is so thin that it is flexible, regarded to be one of the main ingredients of next-generation technologies.
Much of the ongoing research on graphene is focused on developing nanoscale electronic devices. However, scientists note that various technologies—ranging from stretchable electronics to miniature robots so small that they cannot be viewed with the naked eye—necessitate insights into the mechanics of graphene, specifically how it flexes and bends, to unravel their potential.
The bending stiffness of a material is one of its most fundamental mechanical properties. Even though we have been studying graphene for two decades, we have yet to resolve this very fundamental property. The reason is that different research groups have come up with different answers that span across orders of magnitude.
Edmund Han, Study Co-Author and Materials Science and Engineering Graduate Student, University of Illinois
The researchers found out why earlier research efforts disagreed. “They were either bending the material a little or bending it a lot,” stated Jaehyung Yu, a mechanical science and engineering graduate student and study co-author. “But we found that graphene behaves differently in these two situations. When you bend multilayer graphene a little, it acts more like a stiff plate or a piece of wood. When you bend it a lot, it acts like a stack of papers where the atomic layers can slide past each other.”
What is exciting about this work is that it shows that even though everyone disagreed, they were actually all correct. Every group was measuring something different. What we have discovered is a model to explain all the disagreement by showing how they all relate together through different degrees of bending.
Arend van der Zande, Study Co-Author and Professor of Mechanical Science and Engineering, University of Illinois
To produce the bent graphene, Yu developed individual atomic layers of hexagonal boron nitride (another 2D material) into atomic-scale steps. Then, the graphene was stamped on top of the boron nitride layer. Han used a focused ion beam to cut a slice of material. An electron microscope was used to image the atomic structure of this material to observe where each graphene layer was positioned.
Then, the researchers created a set of equations and simulations to compute the bending stiffness using the graphene bend’s shape.
The team draped several layers of graphene over a step with a height of just one to five atoms, devising an accurate and controlled technique for measuring how the material would bend over the step in various configurations.
In this simple structure, there are two kinds of forces involved in bending the graphene. Adhesion, or the attraction of atoms to the surface, tries to pull the material down. The stiffer the material, the more it will try to pop back up, resisting the pull of adhesion. The shape that the graphene takes over the atomic steps encodes all the information about the material’s stiffness.
Pinshane Huang, Study Co-Author and Professor of Materials Science and Engineering, University of Illinois
The research involved systematic control of precisely how much the material bent and how the graphene properties changed.
“Because we studied graphene bent by different amounts, we were able to see the transition from one regime to another, from rigid to flexible and from plate to sheet behavior,” stated mechanical science and engineering professor Elif Ertekin, who headed the computer modeling part of the study. “We built atomic-scale models to show that the reason this could happen is that the individual layers can slip over each other. Once we had this idea, we were able use the electron microscope to confirm the slip between the individual layers.”
According to the researchers, the new outcomes of the study could be applicable to develop machines that are small and sufficiently flexible to interact with biological material or cells.
“Cells can change shape and respond to their environment, and if we want to move in the direction of microrobots or systems that have the capabilities of biological systems, we need to have electronic systems that can change their shapes and be very soft as well,” stated van der Zande. “By taking advantage of interlayer slip, we have shown that the graphene can be orders of magnitude softer than conventional materials of the same thickness.”
This study was supported by the National Science Foundation, through the Illinois Materials Research Center.