Dec 11 2019
Graphene is a two-dimensional (2D) structure composed of carbon. While this material has excellent optical, electronic, and mechanical characteristics, it did not appear to be well suited for magnetic applications.
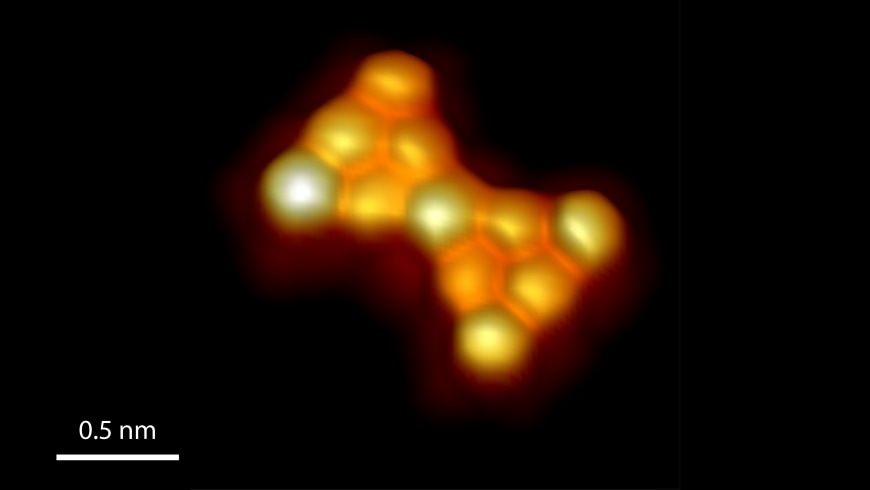 3D-rendered high-resolution scanning tunneling micrograph of Clar’s goblet. Image Credit: Empa.
3D-rendered high-resolution scanning tunneling micrograph of Clar’s goblet. Image Credit: Empa.
Now, Empa scientists, in association with global partners, have successfully synthesized an exclusive nanographene that was predicted back in the 1970s. This nanographene conclusively showed that specific forms of carbon have magnetic characteristics that may lead to spintronic applications in the days to come.
The study results have been recently published in the well-known Nature Nanotechnology journal.
Based on the orientation and shape of their edges, graphene nanostructures (also called nanographenes) can have very different characteristics—for instance, they may display insulating, semiconducting, or conducting behavior. But magnetism is one property that has been elusive to date.
Empa scientists, along with collaborators from Aalto University in Finland, the Technical University in Dresden, University of Bern, and Max Planck Institute for Polymer Research in Mainz, have successfully developed a nanographene that has magnetic characteristics. These characteristics can be a conclusive component for spin-based electronics working at room temperature.
Although graphene contains only carbon atoms, magnetism is a trait that is seldom linked with carbon. Therefore, it is not known how carbon nanomaterials exhibit magnetism. In order to interpret this, the world of atomic physics and chemistry had to be understood first.
In graphene, the carbon atoms are organized in a honeycomb structure. Every carbon atom has three neighbors through which it creates alternating double or single bonds. In the case of a single bond, a single electron from every atom—that is, the so-called valence electron—adheres with its neighbor, whereas in a double bond, a pair of electrons from each atom takes part.
Such an alternating double and single bond representation of organic compounds is called the Kekulé structure. The Kekulé structure was dubbed after August Kekulé, a German chemist who initially suggested this representation for benzene—one of the simplest organic compounds.
In this case, the rule is that pairs of electrons that inhabit the same orbital should vary in their rotational direction—the so-called spin—an outcome of the quantum mechanical principle called Pauli’s exclusion principle.
However, in certain structures made of hexagons, one can never draw alternating single and double bond patterns that satisfy the bonding requirements of every carbon atom. As a consequence, in such structures, one or more electrons are forced to remain unpaired and cannot form a bond.
Shantanu Mishra, PhD Candidate, Empa
Mishra is studying innovative nanographenes in the Empa nanotech@surfaces laboratory led by Roman Fasel. This occurrence of spontaneous unpairing of electrons is referred to as “topological frustration.”
But how is this phenomenon tied with magnetism? The solution lies in the electrons’ “spins.” When an electron rotates around its own axis, it leads to a magnetic moment—a tiny magnetic field. Typically, if there are a pair of electrons with opposite spins in an atom’s orbital, then this magnetic field will cancel one another.
However, if a single electron is alone in its orbital, the magnetic moment will remain and result in a quantifiable magnetic field.
While this phenomenon alone is interesting, one more step is required to effectively utilize the electron spin as circuit elements. One solution could be a structure that resembles a bow tie when looked through a scanning tunneling microscope.
Two Frustrated Electrons in One Molecule
Earlier in the 1970s, Erich Clar, the Czech chemist and a distinguished expert in the area of nanographene chemistry, suggested a bow tie-like structure called “Clar’s goblet.” This structure contains two symmetrical halves and is built such that a single electron in each of the symmetrical halves stays topologically frustrated.
But since both electrons are linked through the structure, they are antiferromagnetically coupled, which means their spins inevitably orient in reverse directions.
Clar’s goblet, in its antiferromagnetic state, can behave as a “NOT” logic gate: if the spin direction at the input is reversed, the spin direction at the output should also be coerced to rotate.
But the structure could not be brought into a ferromagnetic state, where the two spins orient along the same direction. In order to achieve this, the structure should be activated with a specific energy, that is, the so-called exchange coupling energy. This would allow one of the electrons to reverse its spin.
But to remain stable in its antiferromagnetic state, the gate should not change to the ferromagnetic state suddenly. To achieve this, the exchange coupling energy should be greater than the energy dissipation when the gate is being used at room temperature. This is the main prerequisite to make sure that an upcoming spintronic circuit built on nanographenes works seamlessly at room temperature.
From Theory to Reality
But to date, stable magnetic carbon nanostructures at room temperature have merely been theoretical constructs. Now, for the first time, the scientists have successfully developed that structure in practice, and also demonstrated that the theory indeed corresponds to reality.
Realizing the structure is demanding, since Clar’s goblet is highly reactive, and the synthesis is complex.
Shantanu Mishra, PhD Candidate, Empa
Beginning from a precursor molecule, the scientists effectively achieved Clar’s goblet in an ultrahigh vacuum on a surface of gold, and experimentally showed that the molecule has the same properties, as predicted.
Most significantly, the scientists demonstrated that in Clar’s goblet, the exchange coupling energy is comparatively high at 23 meV. This means that spin-based logic operations can thus be stable even at room temperature.
This is a small but important step toward spintronics.
Roman Fasel, Professor, Empa