Dec 16 2019
Researchers have successfully developed a certain type of carbon nanotubes (CNTs) that have 90% selectivity, and have widened the present theory describing the synthesis of these potential nano-cylinders. The study has been recently published in the Science Advances journal.
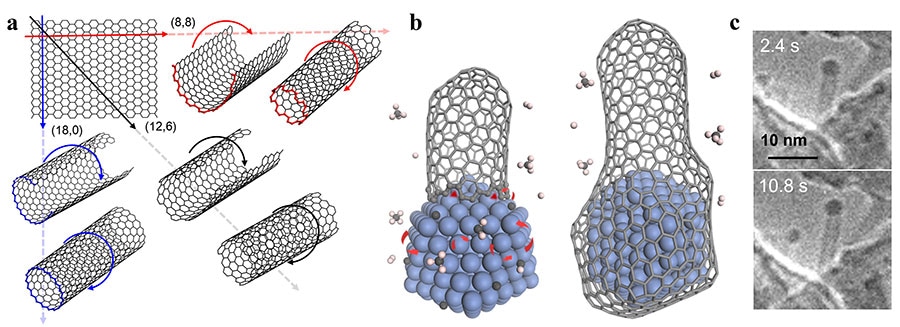 (a) Carbon nanotubes (CNTs) could be viewed as single-atom layer thick graphene sheets rolled into a cylinder. Different directions of rolling determine CNTs’ properties. (b) Schematic diagram showing a carbon nanotube’s lifetime during chemical vapor deposition synthesis. Transition metals (blue structure) serve as catalysts, critical to elongate the CNT (left), until the carbon concentration on the catalyst surface becomes so abundant that the nanoparticle gets encapsulated by graphitic or amorphous carbon, forming a “cap” at the end of the cylinder and ending the growth of the CNT (right). (c) Environmental transmission electron microscope images of a CNT taken at different times during growth. The CNT contains a cobalt nanoparticle on its top end, a typical feature of tip-growth. Image Credit: Institute for Basic Science.
(a) Carbon nanotubes (CNTs) could be viewed as single-atom layer thick graphene sheets rolled into a cylinder. Different directions of rolling determine CNTs’ properties. (b) Schematic diagram showing a carbon nanotube’s lifetime during chemical vapor deposition synthesis. Transition metals (blue structure) serve as catalysts, critical to elongate the CNT (left), until the carbon concentration on the catalyst surface becomes so abundant that the nanoparticle gets encapsulated by graphitic or amorphous carbon, forming a “cap” at the end of the cylinder and ending the growth of the CNT (right). (c) Environmental transmission electron microscope images of a CNT taken at different times during growth. The CNT contains a cobalt nanoparticle on its top end, a typical feature of tip-growth. Image Credit: Institute for Basic Science.
The research was performed by Feng Ding from the Center for Multidimensional Carbon Materials at the Institute for Basic Science (IBS, South Korea) and collaborators.
CNTs are remarkably light yet robust nanomaterials composed of carbon. This carbon has extremely high thermal conductivity and excellent current carrying capacity, which collectively makes the CNTs suitable for use in electronics.
While CNTs are regarded as some of the most fascinating materials for the future, their controllable synthesis still poses a major challenge to researchers. The shape of the CNTs can be compared to paper tubes—just like how a cylinder is produced by rolling a paper sheet, CNTs can be thought of as a single graphite layer rolled up on itself.
In a similar way, different tubes can be created by rolling a sheet of paper around its short side, its long side, or diagonally at varying angles. Based on the direction of rolling, a layer of graphite can create varying CNT structures, where some are semiconducting and others are conducting.
Hence, selective production of a certain type of CNT will be crucial for their upcoming applications, like constructing computer chips that are energy efficient.
However CNTs are not created by the rolling method, but instead, they are grown nanometer after nanometer, while carbon is simultaneously introduced at the edge of nano-cylinders, one atom at a time.
In the last 30 years, numerous studies have been carried out to figure out the growth of CNTs but this understanding remains extremely limited, and practical experimental design for the growth of certain types of CNTs is also very difficult.
Chemical vapor deposition (CVD) is one of the most potential manufacturing techniques developed for CNTs. This process involves combining metal nanoparticles with carbon-containing gases to form CNTs within a high-temperature furnace.
These metal nanoparticles play a major role as catalysts on the tip of the CNTs—the nanoparticles dissociate the carbon source from the carbon-containing gases, and help in binding these carbon atoms to the wall of the CNTs, rendering the tubes increasingly longer. When the catalyst particle is covered by amorphous carbon or graphitic, the CNT growth ceases immediately.
The atoms of carbon are embedded onto the interface between a catalyst nanoparticle and a growing CNT, in the rim’s active sites, and are available to include new atoms. An earlier model of the growth rate of the CNT demonstrated that the former is proportional to the density of the active sites at the CNT-catalyst interface, or the particular CNT structure.
In this analysis, the scientists tracked the stable growth of the CNTs on a magnesium oxide (MgO) support with cobalt nanoparticles as catalysts and carbon monoxide (CO) as the carbon feedstock at 700 °C. The expansion of the earlier theory was demonstrated through direct experimental measurements of 16 CNTs.
It was surprising that the growth rate of a carbon nanotubes only depends on the size of the catalyst particle. This implies that our previous understanding on carbon nanotubes growth was not complete.
Maoshuai He, Study First Author, Institute for Basic Science
In particular, carbon atoms deposited on the surface of catalyst particles can be either removed by etching agents, like CO2, O2, H2O, or H2, or integrated on the CNT’s active side.
To describe the latest experimental observations, the researchers included the impacts of the insertion and removal of carbon at the time of the CNT growth and found that the rate of growth relies on the tube diameter and surface area ratio of the catalyst.
Compared to the previous model, we added three more factors: the rate of precursor deposition, the rate of carbon removal by etching agents, and the rate of carbon insertion into a carbon nanotube wall. When feedstock dissociation cannot be balanced by carbon etching, the rate of carbon nanotube growth will no longer depend on the structure of the carbon nanotube. On the other hand, the previous theory is still valid if the etching is dominating.
Feng Ding, Group Leader, Center for Multidimensional Carbon Materials, Institute for Basic Science
Fascinatingly, the latest concept of CNT growth resulted in a novel mechanism to selectively grow a particular type of CNTs, represented as (2n, n) CNTs, characterized by the highest number of active sites present at the CNT-catalyst interface. This structure of CNT would relate to rolling a graphite sheet diagonally at an angle of about 19°.
If there is no carbon etching and the carbon nanotubes growth is slow, carbon atoms on the catalyst surface will accumulate. This may lead to the formation of graphitic or amorphous carbon, which are established mechanisms of carbon nanotube growth termination. In this case, only carbon nanotubes which are able to add carbon atoms on their walls, that is with the highest number of active sites, can survive.
Jin Zhang, Study Co-Author and Professor, Peking University, China
In line with the latest theoretical interpretation, the scientists successfully designed experiments that yielded (2n, n) CNTs with up to 90% selectivity: the maximum selective growth of this kind of CNT was accomplished with a high concentration of feedstock and without using any etching agent.