Feb 10 2020
At the Tokyo Institute of Technology (Tokyo Tech), researchers have demonstrated that sub-nanoscale copper oxide particles are more potent catalysts compared to those on the nanoscale.
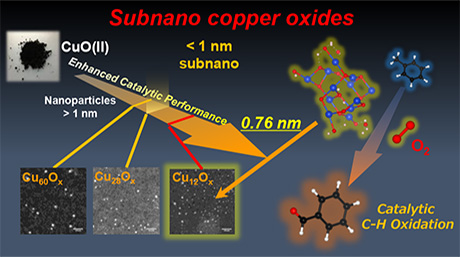 A research concept: Sub-nano copper oxide particles for solvent-free aerobic oxidation of hydrocarbons. Image Credit: Tokyo Institute of Technology.
A research concept: Sub-nano copper oxide particles for solvent-free aerobic oxidation of hydrocarbons. Image Credit: Tokyo Institute of Technology.
Moreover, when compared to existing catalysts used in industry, these sub-nanoparticles have the ability to catalyze the oxidation reactions of aromatic hydrocarbons much more effectively. This research opens the door to improved and more efficient use of aromatic hydrocarbons, which are crucial materials for both industry and research.
In various industrial processes and chemical reactions, the selective oxidation of hydrocarbons is critical. Therefore, researchers have been seeking more efficient techniques to perform this oxidation. It has been found that copper oxide (CunOx) nanoparticles are useful as a catalyst to process aromatic hydrocarbons, but there has been an ever-growing demand for even more effective compounds.
In the last few years, researchers used catalysts based on noble metals, including particles at the sub-nano level. At this level, the size of the particles is less than 1 nm. When such particles are placed on suitable substrates, they can provide much higher surface areas compared to nanoparticle catalysts to boost the reactivity.
Along these lines, a research team from Tokyo Tech, including Prof. Kimihisa Yamamoto and Dr Makoto Tanabe, studied chemical reactions catalyzed by CunOx sub-nanoparticles (SNPs) to assess their performance in oxidizing aromatic hydrocarbons.
CunOx SNPs with three particular sizes (including 12, 28, and 60 copper atoms) were synthesized within tree-like frameworks known as dendrimers. They were supported on a zirconia substrate and applied to carry out the aerobic oxidation of an organic compound comprising an aromatic benzene ring.
Using infrared (IR) spectroscopy and X-ray photoelectron spectroscopy (XPS), the researchers investigated the structures of the produced SNPs. The results were validated by density functionality theory (DFT) calculations.
The DFT calculations and XPS analysis showed a rise in ionicity of the copper–oxygen (Cu–O) bonds with a decrease in the SNP size. This bond polarization was found to be higher compared to what was observed seen in bulk Cu–O bonds. Moreover, the greater polarization was due to the improved catalytic activity of the CunOx SNPs.
Tanabe and the team noticed that the CunOx SNPs expedited the oxidation of the CH3 groups bound to the aromatic ring. This led to the formation of products. No products were produced when the CunOx SNP catalyst was not utilized. The catalyst that had the smallest CunOx SNPs (Cu12Ox) had the most superior catalytic performance and was found to be most durable.
The enhancement of the ionicity of the Cu–O bonds with decrease in size of the CunOx SNPs enables their better catalytic activity for aromatic hydrocarbon oxidations.
Dr Makoto Tanabe, Researcher, Tokyo Institute of Technology
Their study confirms the hypothesis that copper oxide SNPs are highly promising as catalysts in industrial applications. Yamamoto suggested the possible future applications of CunOx SNPs.
The catalytic performance and mechanism of these size-controlled synthesized CunOx SNPs would be better than those of noble metal catalysts, which are most commonly used in industry at present.
Kimihisa Yamamoto, Professor, Tokyo Institute of Technology