Jun 15 2020
A new technique has been developed by scientists at Carnegie Mellon University for self-assembling nanostructures with gamma-modified peptide nucleic acid (γPNA)—an artificial imitator of DNA.
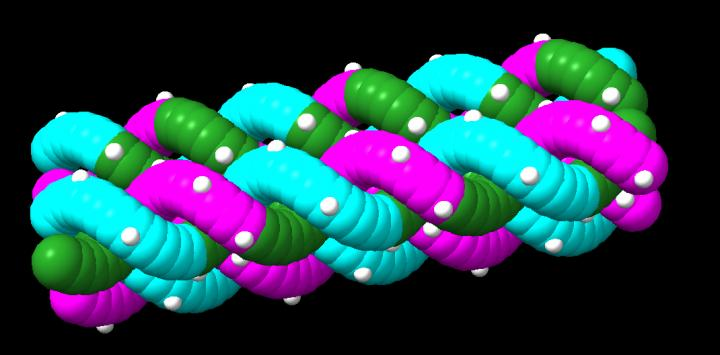 The above image is a representation that shows interwoven gamma-modified PNA oligomers. Gamma modifications (shown in white) uniformly decorate the structure, increasing its binding strength and providing chemical modification. Image Credit: College of Engineering, Carnegie Mellon University.
The above image is a representation that shows interwoven gamma-modified PNA oligomers. Gamma modifications (shown in white) uniformly decorate the structure, increasing its binding strength and providing chemical modification. Image Credit: College of Engineering, Carnegie Mellon University.
Such a process may have an impact on nanomanufacturing, and also on upcoming biomedical technologies such as drug delivery and targeted diagnostics.
The study, recently published in the Nature Communications journal, introduces a scientific concept of γPNA nanotechnology that allows the self-assembly in organic solvent solutions—the aggressive environments used in the synthesis of polymers and peptides. This technology offers excellent potential for nanosensing and nanofabrication.
Headed by Rebecca Taylor, an assistant professor of mechanical engineering, the scientists have reported that γPNA is capable of forming nanofibers in organic solvent solutions and these tiny fibers can grow up to a length of 11 µm (over 1000 times longer than their thickness). These findings indicate the first-ever complex, all-PNA nanostructures to be grown in organic solvents.
Taylor, who leads the Microsystems and MechanoBiology Lab at Carnegie Mellon University, wishes to exploit the “superpowers” of PNA. γPNA not only exhibits higher thermal stability but also has the potential to attach to other kinds of nucleic acids present in organic solvent mixtures that would often destabilize the structural DNA nanotechnology.
This implies that γPNA can form nanostructures in solvent settings that inhibit the formation of nanostructures based on DNA.
Another characteristic of γPNA is that it is less twisted when compared to that of the double helix of DNA. The outcome of this variation is that the “rules” for engineering PNA-based nanostructures are entirely different than the rules for engineering structural DNA nanotechnology.
As mechanical engineers, we were prepared for the challenge of solving a structural design problem. Due to the unusual helical twist, we had to come up with a new approach for weaving these pieces together.
Rebecca Taylor, Assistant Professor, Department of Mechanical Engineering, Carnegie Mellon University
Since the scientists working in Taylor’s laboratory are looking for ways to apply the dynamic shape change in their γPNA nanostructures, they were fascinated to find that morphological variations—similar to unraveling or stiffening—took place when they integrated the DNA into the γPNA nanostructures.
The scientists also want to further explore other fascinated properties, such as aggregation and solubility in water. These current nanofibers in water are likely to combine together. In organic solvent mixtures, the Taylor laboratory has shown that it can control the aggregation of structures, and according to Taylor, the aggregation is a characteristic that can be exploited.
These nanofibers follow the Watson-Crick binding rules of DNA, but they appear to act more and more like peptides and proteins as PNA structures grow in size and complexity. DNA structures repel each other, but these new materials do not, and potentially we can leverage this for creating responsive surface coatings.
Rebecca Taylor, Assistant Professor, Department of Mechanical Engineering, Carnegie Mellon University
Recognized as a simple DNA mimic, the synthetic γPNA molecule has the required characteristics, like strong affinity and high biostability for complementary nucleic acids.
We believe through this work, we could additionally adjust this perception by highlighting the ability of γPNA to act as both—as a peptide mimic because of its pseudopeptide backbone and as a DNA mimic because of its sequence complementarity. This change in perception could allow us to understand the multiple identities this molecule can leverage in the world of PNA nanostructure design.
Sriram Kumar, Study First Author and Mechanical Engineering PhD Candidate, Department of Mechanical Engineering, Carnegie Mellon University
While the PNA technology is currently being employed in innovative gene therapy applications, there is much to learn about the potential of this synthetic material.
If there is a scope to form complex PNA nanostructures in aqueous solutions, then more applications will comprise enzyme-resistant nanomachines, such as nanorobots, diagnostics, and biosensors, believes Taylor’s research team.
“PNA-peptide hybrids will create a whole new toolkit for scientists,” added Taylor.
The scientists employed custom gamma alterations to PNA created by Danith Ly’s laboratory at Carnegie Mellon. Going forward, future studies will analyze left-handed γPNAs in the nanomanufacturing procedure. For upcoming biomedical applications, left-handed structures are expected to be specifically fascinating because they would not likely attach to cellular DNA.
The study represents an interdisciplinary association. Other authors of the study include chemistry PhD candidate Alexander Pearse and mechanical engineering candidate Ying Liu.
The work was financially supported by the Air Force Office of Science Research and the National Science Foundation.
Journal Reference:
Kumar, S., et al. (2020) Modular self-assembly of gamma-modified peptide nucleic acids in organic solvent mixtures. Nature Communications. doi.org/10.1038/s41467-020-16759-8.
Rebecca Taylor: Making Better Nanostructures from Synthetic DNA
Video Credit: College of Engineering, Carnegie Mellon University.