Aug 3 2020
A team of researchers from the Tokyo Institute of Technology has investigated an innovative yet simple technique to produce manganese dioxide that has a particular crystalline structure known as β-MnO2.
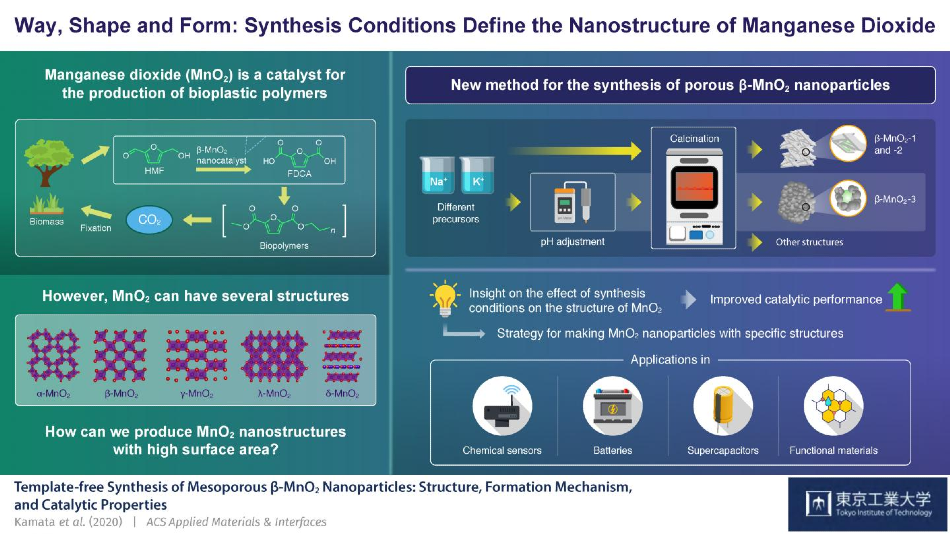 Scientists at the Tokyo Institute of Technology explore a novel and simplistic method to synthesize manganese dioxide with a specific crystalline structure called ß-MnO2. Their study sheds light on how different synthesis conditions can produce manganese dioxide with distinct porous structures, hinting at a strategy for the development of highly tuned MnO2 nanomaterials that could serve as catalysts in the fabrication of bioplastics. Image Credit: Keigo Kamata, Tokyo Institute of Technology.
Scientists at the Tokyo Institute of Technology explore a novel and simplistic method to synthesize manganese dioxide with a specific crystalline structure called ß-MnO2. Their study sheds light on how different synthesis conditions can produce manganese dioxide with distinct porous structures, hinting at a strategy for the development of highly tuned MnO2 nanomaterials that could serve as catalysts in the fabrication of bioplastics. Image Credit: Keigo Kamata, Tokyo Institute of Technology.
The study provides a better understanding of how different conditions of synthesis can yield manganese dioxide that has distinct porous structures, offering clues for developing extremely modified MnO2 nanomaterials that can potentially act as catalysts in the development of bioplastics.
Materials engineering has progressed to such an extent that individuals are concerned about the chemical make-up of a material and also about its structure at a nanometric scale.
Recently, nanostructured materials have attracted a great deal of interest from scientists working on a wide range of domains and for good reason; the electrical, optical, and physical properties of the nanostructured materials can be adjusted and driven to the limit as soon as techniques to customize their nanostructure are available.
Manganese dioxide, whose chemical formula is MnO2, is a nanostructured metal oxide that is capable of forming several different crystalline structures. It is used across numerous engineering fields.
Among these, MnO2 is mainly used as a catalyst for chemical reactions, and β-MnO2—a specific crystalline structure of MnO2—is exceptional for oxidizing 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid, or FDCA.
Since FDCA can be used for creating eco-friendly bioplastics, new methods should be identified to adjust the nanostructure of β-MnO2 to boost its catalytic performance.
But it is difficult to create β-MnO2 when compared to other crystalline structures of MnO2. Prevalent techniques are not only complex but also require the use of template materials. β-MnO2 “grows” on these template materials and ends up with the required structure following many steps.
Now, a research team from the Tokyo Institute of Technology and headed by Professor Keigo Kamata has investigated a template-free method for producing different kinds of porous β-MnO2 nanoparticles.
The researchers’ technique, detailed in the study published in the ACS Applied Materials & Interfaces journal, is remarkably simple and easy. Precursors of manganese are initially acquired by combining aqueous solutions and allowing the solids to precipitate.
Once the filtration and drying procedures are completed, the solids, thus collected, are exposed to a temperature of 400 °C in regular atmospheric air, a procedure called calcination. At the time of this process, the material crystallizes and the resultant black powder is over 97% porous β-MnO2.
Most significantly, the scientists discovered that when this porous β-MnO2 is used as a catalyst, it is relatively more efficient for producing FDCA when compared to the β-MnO2 synthesized using a more extensive technique, known as the “hydrothermal method.”
To find out the reason behind this, the researchers studied the spectral, microscopic, and chemical properties of β-MnO2 nanoparticles created under varying synthesis conditions.
The team found that β-MnO2 can assume strikingly different morphologies in accordance with specific parameters. Particularly, scientists can obtain MnO2 nanoparticles with huge spherical pores by simply altering the acidity (pH) of the solution, where the precursors are combined. A porous structure like this has a higher surface area, thereby offering a more improved catalytic performance.
Kamata was excited about the outcomes.
Our porous β-MnO2 nanoparticles could efficiently catalyze the oxidation of HMF into FDCA in sharp contrast with β-MnO2 nanoparticles obtained via the hydrothermal method. Further fine control of the crystallinity and/or porous structure of β-MnO2 could lead to the development of even more efficient oxidative reactions.
Keigo Kamata, Professor, Tokyo Institute of Technology
In addition, the latest study offered a greater understanding of the formation of tunnel and porous structures in MnO2, which could be crucial to extend its applications.
Our approach, which involves the transformation of Mn precursors into MnO2 not in the liquid-phase (hydrothermal method) but under an air atmosphere, is a promising strategy for the synthesis of various MnO2 nanoparticles with tunnel structures. These could be applicable as versatile functional materials for catalysts, chemical sensors, lithium-ion batteries, and supercapacitors.
Keigo Kamata, Professor, Tokyo Institute of Technology
Such additional studies are expected to tap the entire potential of nanostructured materials in the days to come.
Journal Reference:
Yamaguchi, Y., et al. (2020) Template-Free Synthesis of Mesoporous β-MnO2 Nanoparticles: Structure, Formation Mechanism, and Catalytic Properties. ACS Applied Materials & Interfaces. doi.org/10.1021/acsami.0c08043.