Oct 5 2020
The heterogeneity of cancer cells themselves is one of the main difficulties faced in the development of effective and targeted treatments for cancer. This change makes it hard for the immune system to identify, react to, and actively fight against tumors.
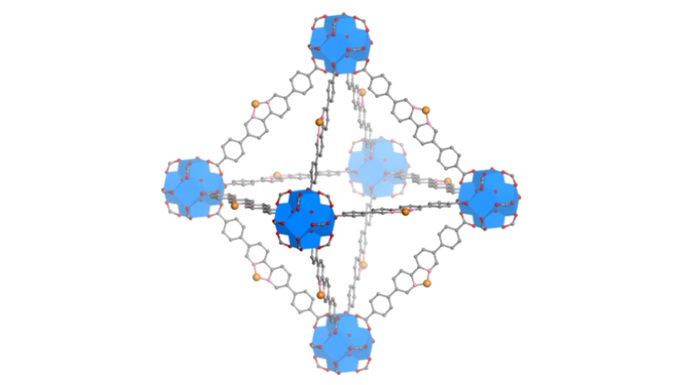 A model of the new nanoscale metal-organic framework that can generate free radicals within tumor tissue using X-rays to kill cancer cells directly, and can be loaded with immune signaling molecules to activate the immune response against tumor cells. Image Credit: Guangxu Lan in the Lin Group.
A model of the new nanoscale metal-organic framework that can generate free radicals within tumor tissue using X-rays to kill cancer cells directly, and can be loaded with immune signaling molecules to activate the immune response against tumor cells. Image Credit: Guangxu Lan in the Lin Group.
But new advances in the field of nanotechnology are currently rendering it feasible to provide targeted and personalized “vaccines” to treat cancer.
A new study, reported recently in the Science Advances journal, describes the use of charged nanoscale metal-organic frameworks and X-rays to produce free radicals inside tumor tissue to directly destroy the cancer cells.
Moreover, it is possible to use the same frameworks to provide immune signaling molecules called PAMPs to trigger the immune response against tumor cells. By integrating these two methods into one “vaccine” that can be easily administered, this new technology could pave the way to improve local and systemic treatment of cancers that are hard to treat.
As part of a collaboration between the Lin Group from the Department of Chemistry at the University of Chicago and the Weichselbaum Lab at the University of Chicago Medicine, the researchers combined expertise from cancer biology and inorganic chemistry to address the challenging issue of correctly targeting and triggering an innate immune response against cancer.
This study took advantage of the special properties of nanoscale metal-organic frameworks (nMOFs), which are nanoscale structures made of recurring units arranged in a lattice formation and with the ability to infiltrate tumors.
Such nMOFs can be irradiated with the help of X-rays to produce high concentrations of free oxygen radicals, thereby directly killing the cancer cells and generating inflammatory molecules and antigens helping the immune system identify and clear cancerous cells, quite similar to a vaccine.
Moreover, the lattice-like structure of the nMOFs makes them ideal transporters to directly deliver anti-cancer drugs to tumors. But to date, it has been challenging to trigger innate and adaptive immune responses essential for destroying cancerous tumors.
As part of the new research, the team further refined their technique. They produced a new kind of nMOF structure that can be loaded with drugs called pathogen-associated molecular patterns (PAMPs).
Upon applying the nMOFs to cancerous tumors, irradiation of the tissue had a double impact: it activated the nMOFs to destroy local cancer cells to create antigens against the tumor and discharged the PAMPs, which subsequently triggered a much more powerful activation of the immune response to the tumor antigens.
This one-two punch could destroy both pancreatic and colon cancer cells with high efficacy, even in tumor models that are considerably resistant to other types of immunotherapy.
The investigators performed additional experiments with mice and observed that they could expand the impacts of the nMOFs even to distant tumors by applying checkpoint inhibitors, offering new hopes for treating cancer both systemically and locally with this method.
By including PAMP delivery with the nMOFs, this is the first time we were able to really enhance the immune response to the antigens. This is entirely different from all of our previous studies because we’ve shown that the nMOFs plus PAMPs can impact all of the aspects required for activating the immune system.
Wenbin Lin, PhD, Study Senior Author and James Franck Professor of Chemistry, University of Chicago Medical Center
“We can use this nanoformulation to enable personalized cancer vaccinations that will work on any patient because this strategy will not be subject to the heterogeneity we see among different patients,” added Lin.
The impacts of the treatment were so marked that the scientists are keen to bring the technology to clinical trials, whereas the other versions of the nMOF technology are already being tested, with encouraging results until now.
The brilliance of this system is twofold. First, it can improve local tumor control by increasing the killing power of X-rays. Second, while there has been interest in using radiation to stimulate the immune response to fight cancer, it has turned out to be harder than we thought.
Ralph Weichselbaum, MD, Study Co-Author and Daniel K. Ludwig Distinguished Service Professor of Radiation and Cellular Oncology, University of Chicago Medical Center
“In this case, the nMOFs are able to activate the innate and adaptive immune systems, which makes this technology very promising for treating cancer in the clinic,” added Weichselbaum, who is also the Chair of the Department of Radiation and Cellular Oncology at UChicago.
The investigators are already looking forward to next steps and striving to fine-tune the technology. “We’re refining the design of the nMOF and its delivery of the PAMPs, in preparation for testing it in humans,” stated Lin. “We’re really working on zooming in on the best formulation so we can get this into clinical trials, hopefully in the next two to three years, or even sooner.”
The researchers acknowledge the interdisciplinary and collaborative nature of UChicago and the University of Chicago Medicine’s Hyde Park campus for making a space where cancer biology and chemistry have combined to create such an encouraging potential therapy, as well as the support they have received from Ludwig Cancer Research along the way.
From the conception of this project and getting it funded to starting with clinical trials where we’re able to test the technology in clinical trials and get real patient data, this work has all been done right here at UChicago. We really are going from discovering something in the lab to testing it at the bedside.
Ralph Weichselbaum, MD, Study Co-Author and Daniel K. Ludwig Distinguished Service Professor of Radiation and Cellular Oncology, University of Chicago Medical Center
The study titled “Nanoscale metal-organic frameworks for x-ray activated in situ cancer vaccination” was financially supported by the National Cancer Institute (U01-CA198989 and 1R01CA253655), the Department of Defense (PC170934P2), the University of Chicago Medicine Comprehensive Cancer Center (NIH CCSG: P30 CA014599), and the Ludwig Institute for Metastasis Research.
The additional authors of the study are Kaiyuan Ni, Guangxu Lan, Nining Guo, August Culbert, Taokun Luo, and Tong Wu of the University of Chicago.
Journal Reference:
Ni, K., et al. (2020) Nanoscale metal-organic frameworks for x-ray activated in situ cancer vaccination. Science Advances. doi.org/10.1126/sciadv.abb5223.