May 27 2021
A new study from the University of Surrey, in collaboration with the University of Cambridge and Graz University of Technology (Austria), reveals that energy is needed for water to proceed through the first step of ice formation on graphene.
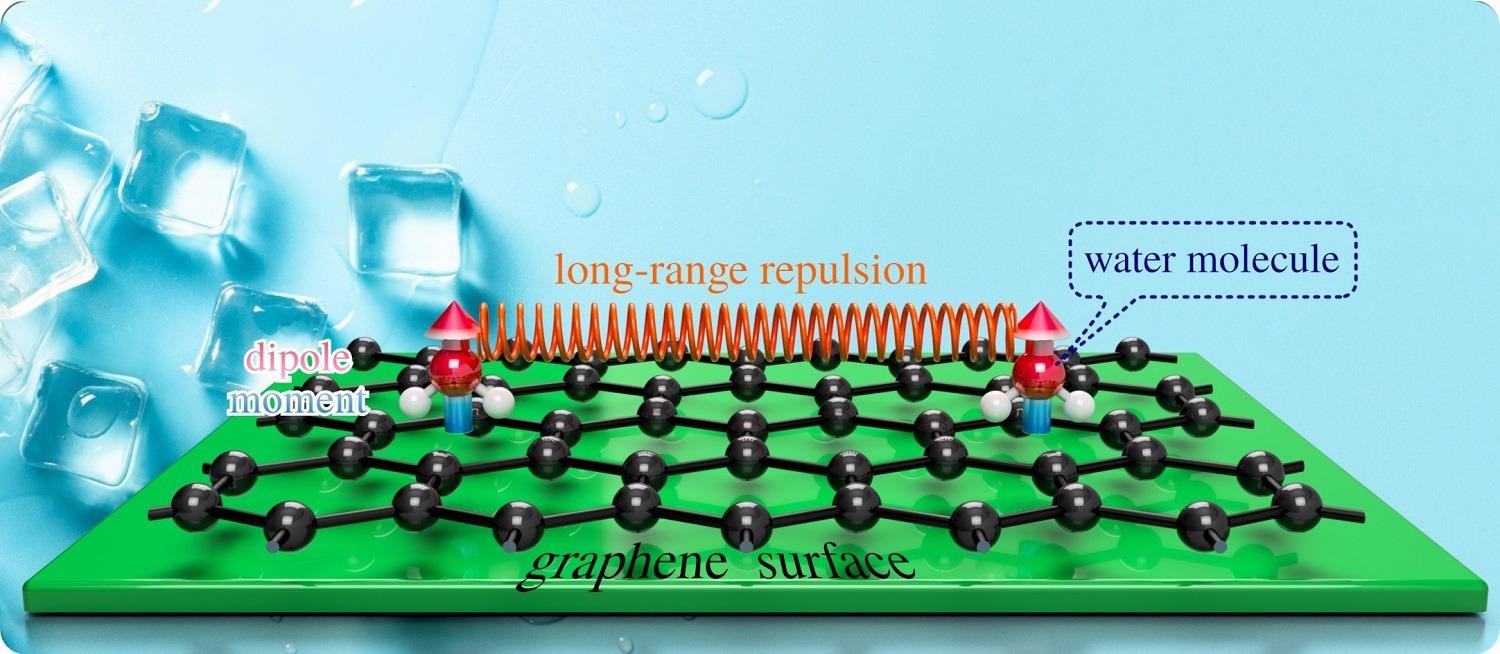 Image Credit: University of Surrey
Image Credit: University of Surrey
In a paper published in Nature Communications, the research team details the complex physical processes at work to understand the chemistry of ice formation. The molecular-level perspective of this process may help in predicting the formation and melting of ice, from individual crystals to glaciers and ice sheets. The latter being crucial to quantify environmental transformation in connection with climate change and global warming [1].
The team was able to track down the first step in ice formation, called nucleation, which happens in an incredibly short length of time, a fraction of a billionth of a second, when highly mobile individual water molecules find each other and coalesce. However, conventional microscopes are far too slow to follow the motion of water molecules, so it is impossible to use them to monitor how molecules combine on top of solid surfaces.
The research team used a state-of-the-art Helium Spin-Echo (HeSE) machine to follow the motion of atoms and molecules. The team used HeSE to study the motion of water molecules on a model pristine graphene surface [3,4]. The researchers made a remarkable observation: the water molecules repel each other and need to gain sufficient energy to overcome that repulsion before ice can start to form.
It is the combination of both experimental and theoretical methods that have allowed the international team of scientists to unravel the behaviour of the water molecules. Together these have captured, for the first time, exactly how the first step of ice formation at a surface evolves and allows them to propose a previously unknown physical mechanism.
Dr Marco Sacchi, co-author of the study and Royal Society University Research Fellow at the University of Surrey, said: “Our results show that water molecules need to overcome a small but important energy barrier before forming ice. We hope that our unique collaborative project will go some way to helping us all understand the dramatic changes that are happening right across our planet.”
Dr Anton Tamtögl, lead and corresponding author, from Graz University of Technology, adds: “The observations completely alter our understanding of ice nucleation. The HeSE results looked very promising, but the motion of water was incredibly complicated and suggested counter-intuitive new physics. We decided that atomistic simulations were needed to interpret the results.”
Dr Andrew Jardine a Reader in Experimental Physics from the University of Cambridge, one of the developers of the HeSE method, said: “The technique is completely revolutionising our ability to follow physical and chemical processes at the single molecule level.”
Dr Bill Allison, also from the University of Cambridge, said: “Repulsion between water molecules has simply not been considered during ice nucleation - this work will change all that. The newly observed interactions also change the rate at which nucleation takes place, and hence at which ice can form. The work will therefore have important consequences in preventing ice formation, which is relevant to fields as diverse as wind power, aviation and telecommunications.”
References
- https://climate.nasa.gov/vital-signs/ice-sheets/
- Cambridge Atom Scattering Centre, https://atomscattering.phy.cam.ac.uk
- A. Tamtögl et al., Motion of water monomers reveals a kinetic barrier to ice nucleation on graphene. Nat. Commun. 12 3120 (2021).
- “Behind the paper” https://chemistrycommunity.nature.com/posts/it-takes-some-heat-to-form-ice