Jun 1 2021
For the first time, an interdisciplinary group from the Max Planck Institute of Colloids and Interfaces has developed a laser-driven technology that allows them to make nanoparticles like nickel oxides, cobalt, and copper.
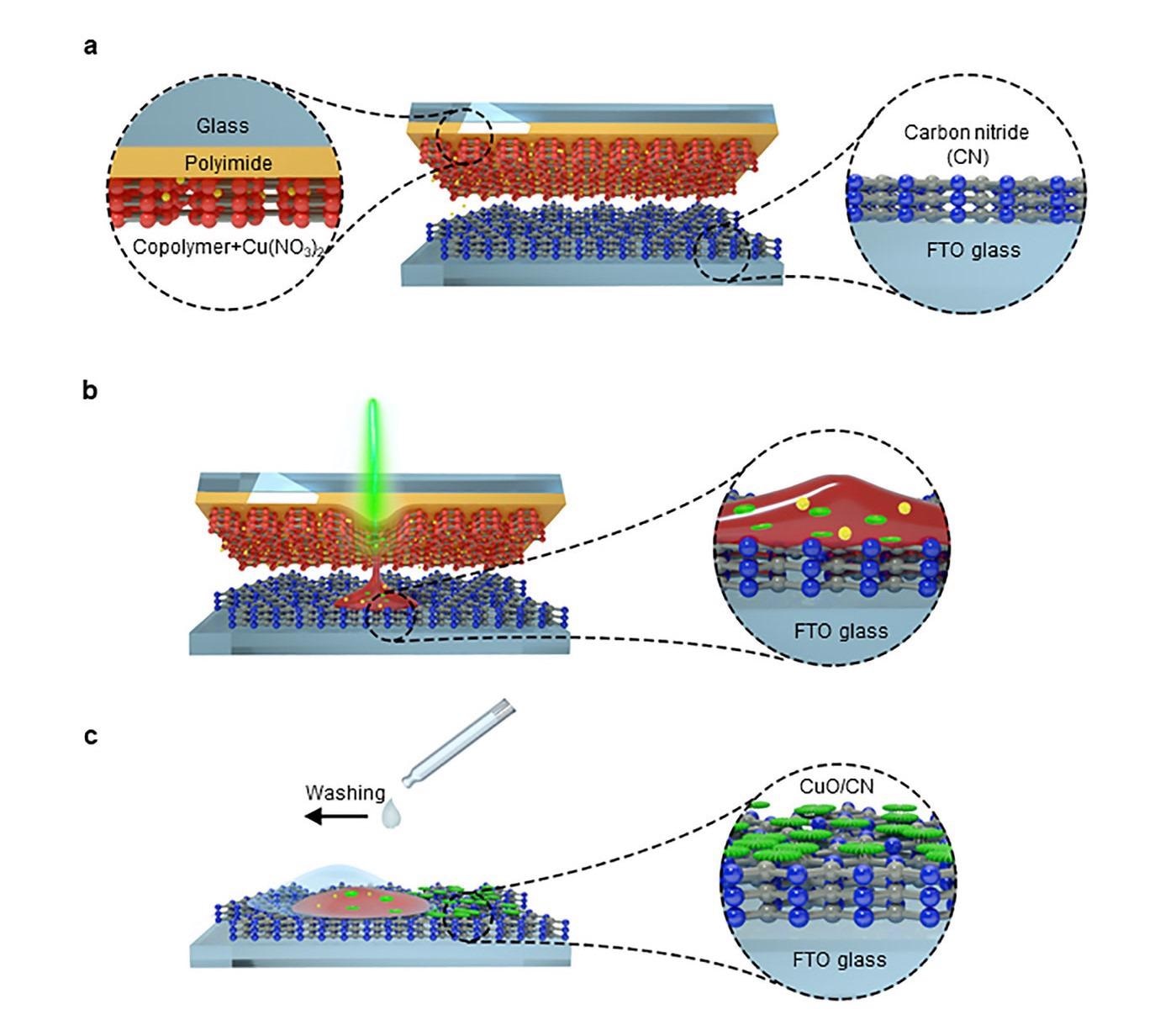 This is how the new laser-driven method works. Image Credit: Max Planck Institute of Colloids and Interfaces.
This is how the new laser-driven method works. Image Credit: Max Planck Institute of Colloids and Interfaces.
The study was published in the Nature Communications journal.
Photoelectrodes have been generated through this method at normal printing speed, for instance, for an extensive range of applications like the production of green hydrogen.
Early techniques synthesized such nanomaterials with only high energy input in conventional reaction vessels and took several hours. The laser-powered technology devised at the institute can help the researchers deposit small quantities of material on a surface and concurrently carry out chemical synthesis in a very short period of time by making use of high temperatures from the laser.
When I discovered the nanocrystals under the electron microscope, I knew I was onto something big.
Junfang Zhang, Study First Author and Doctoral Researcher, Max Planck Institute of Colloids and Interfaces
The breakthrough became a new and eco-friendly technique for synthesizing materials that have the ability to efficiently convert solar energy into electricity amongst other things. But it achieves it without detours with sunlight to hydrogen, stated Savateev.
Nowadays most of green hydrogen is produced from water using electricity generated by solar panels and stored in batteries. By employing photoelectrodes we can use solar light directly.
Dr Aleksandr Savateev, Max Planck Institute of Colloids and Interfaces
The new concept applies to what are called transition metal oxides, mostly nickel oxides, cobalt, and copper, all of which are considered to be good catalysts. The unique feature of these oxides is the range of their crystal forms (nanocrystals like nanostars or nanorods ), which impact their surface energy. Every structure can exert a different impact on catalytic reactions.
Thus, it is essential that these nanostructures can be made targeted, or even untargeted, yet repeatable. Moreover, the developed technology could be utilized to identify new catalysts rapidly and efficiently.
Laser dot by laser dot, we can create different catalysts side by side by simply varying the composition and conditions, and then also test them in parallel right away. But now we need to work on making the catalyst systems more persistent in all applications.
Dr Felix Löffler, Max Planck Institute of Colloids and Interfaces
The Method
Equivalent to the principle of a typewriter, the material is shifted from a donor to an acceptor carrier. On the former is the “ink,” a solid polymer, which is blended with metal salts, and the latter comprises a thin carbon nitride film on a conductive electrode.
Targeted laser irradiation carries the salts to the acceptor together with the molten polymer. The short-time high temperatures make the salts react in a few milliseconds and they change into metal oxide nanoparticles with the preferred morphology.
Journal Reference:
Zhang, J., et al. (2021) Laser-driven growth of structurally defined transition metal oxide nanocrystals on carbon nitride photoelectrodes in milliseconds. Nature Communications. doi.org/10.1038/s41467-021-23367-7.