Jun 29 2021
At Nagoya University, Japan, a research group has developed a new technique for synthesizing nanographenes, a type of nanocarbon with huge potential as a next-generation material, in a quick and efficient way.
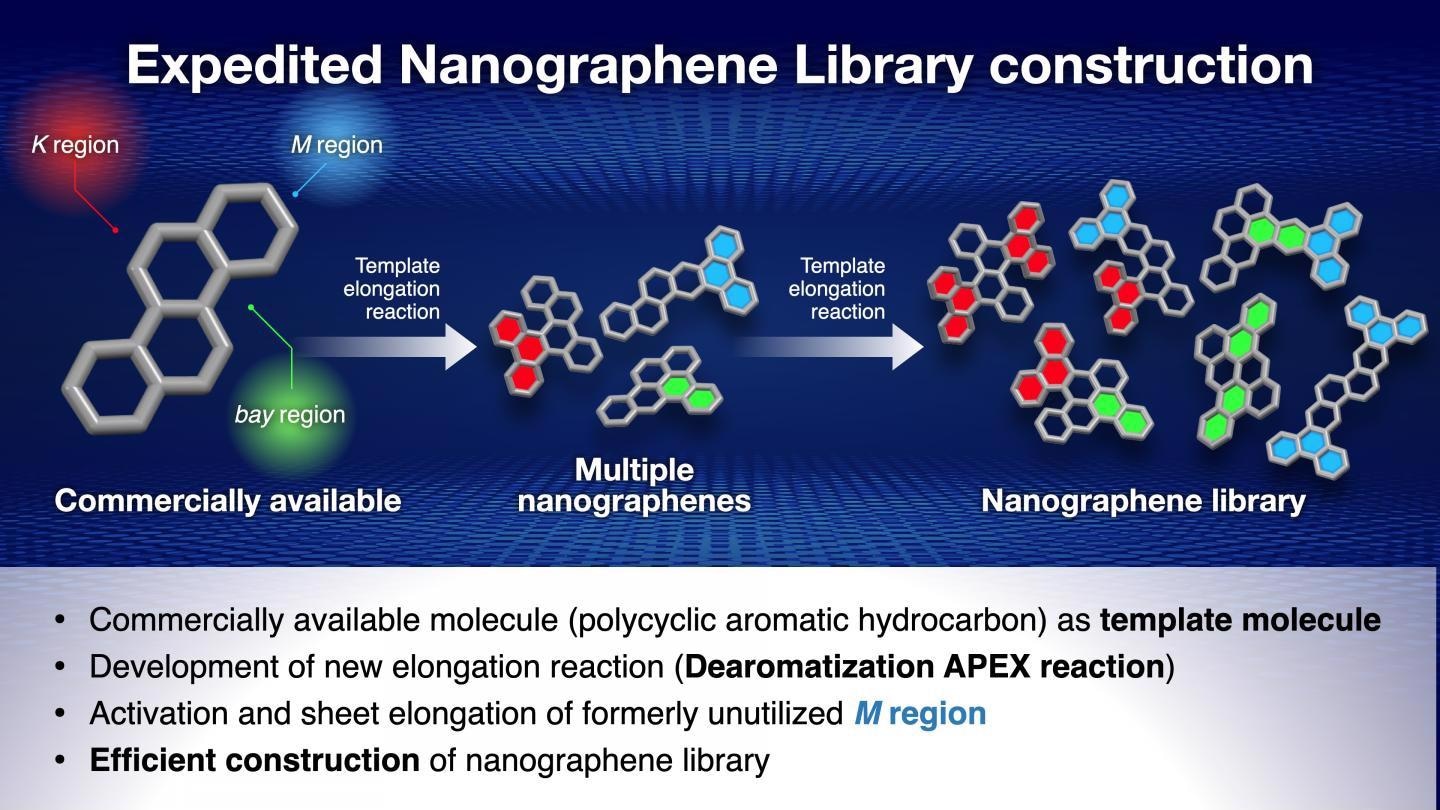 APEX reactions are carried out on the K, M, and bay regions of the polycyclic aromatic hydrocarbon, synthesizing multiple nanographenes. These reactions can then be repeated, further increasing the number of potential nanographene structures that can be synthesized. Image Credit: Issey Takahashi.
APEX reactions are carried out on the K, M, and bay regions of the polycyclic aromatic hydrocarbon, synthesizing multiple nanographenes. These reactions can then be repeated, further increasing the number of potential nanographene structures that can be synthesized. Image Credit: Issey Takahashi.
Nanographenes are graphene’s part structures, which is a sheet of carbon atoms with a thickness of around 3 nm and specific potential for use in semiconductor development. They have electron mobility that is several hundred times higher compared to this generation materials.
Graphene was isolated in 2004 for the first time, a breakthrough that fetched the 2010 Nobel Prize in physics, making it a very novel material which is considered to be the subject of a great deal of study.
Researchers exhibit comparable interest in nanographenes in the nanocarbon research field since they exhibit electric and magnetic characteristics beyond those of graphene. The biggest barrier, although it is an exciting one, that has been experienced by scientists is the number of possible nanographenes.
The number of potentially possible nanographene structures increases with the number of benzene rings (6 atoms of carbon in a hexagonal formation) that make them. For instance, even a comparatively small 10 benzene ring nanographene might possess up to 16,000 variants.
Since each nanographene exhibits different physical characteristics, the key to applied nanographene study is to determine the relationship between the structure and characteristics of as many nanographenes as possible.
The task of the researchers is thus to make a nanographene library, including data on the properties of as many nanographenes as possible. Yet, the present technique of nanographene synthesis, called a coupling reaction, is a multi-step process that generates an individual nanographene.
Therefore, for a 100-nanographene library to be created, the execution of 100 individual coupling reactions is essential. Even this would be a significant undertaking, making the construction of a truly extensive nanographene library virtually impossible.
To address this issue, the Nagoya University researchers, headed by Professor Kenichiro Itami, worked on the APEX reaction. It is a reaction that involves using polycyclic aromatic hydrocarbons as templates for producing nanographenes.
Polycyclic aromatic hydrocarbons include three regions of their structure — called the bay region, M region and K region — which can be elongated in an APEX reaction, thereby generating three nanographenes.
These nanographenes can then be elongated further in another reaction, implying that a huge number of nanographenes could be synthesized from a single polycyclic aromatic hydrocarbon template molecule.
Professor Itami’s team has already developed the K region APEX reaction and another group of researchers has done so for the bay region. So, the focus was on the M region.
The researchers activated the M region with the help of the 1950 Nobel Prize-winning Diels-Alder reaction and were successful in performing an elongation reaction on the activated M region. This enabled all three viable sites on the polycyclic aromatic hydrocarbons proficient to synthesize nanographenes.
The scientists could generate 13 nanographenes along with three APEX reactions, with a majority of them being new structures, thereby proving both the versatility and efficiency of this new technique.
This fascinating new study and its ability to expedite the making of nanographene libraries is a step towards the advent of the next generation of materials with the ability to revolutionize solar energy and semiconductors and enhance lives globally.
Journal Reference:
Matsuoka, W., et al. (2021) Diversity-oriented synthesis of nanographenes enabled by dearomative annulative π-extension. Nature Communications. doi.org/10.1038/s41467-021-24261-y.