An exciting review has outlined the significance of mitochondrial function for neuroinflammatory and neurodegenerative diseases. This research has been published in the journal Pharmaceutics, with the aim of uncovering the impact mitochondrial dysfunction has on the overall unbalance within cells.
Study: Nanotechnology-Based Drug Delivery Strategies to Repair the Mitochondrial Function in Neuroinflammatory and Neurodegenerative Diseases. Image Credit: 3d_man/Shutterstock.com
The researchers discussed different strategies for selective mitochondrial ligand targeting and novel solutions involving nanomaterials and drug-loaded nanosystems. These approaches can be developed to repair mitochondrial dysfunction against oxidative stress, preventing cell death, and therefore improve motor and cognitive disability.
How Is Mitochondria Important for Neuroinflammatory and Neurodegenerative Diseases?
Mitochondria hold a significant function in cells. In eukaryotic cells, these vital organelles control a range of physiological processes associated with energy production and cellular processes, such as cell death, and calcium homeostasis. Mitochondria are also involved in generating and mediating reactive oxygen species (ROS), an important component for oxidative stress.
Mitochondrial dynamics refer to coordinated cycles of fission and fusion that control the shape, distribution, and size of mitochondria.
Damaged mitochondria are removed through a process called mitophagy. Mitophagy includes selective degradation of mitochondria through autophagy when mitochondria organelles become defective after being damaged or stressed.
These processes are critical for functional mitochondria, and any abnormalities or imbalances which affect these processes can have negative consequences to their biology as well as the viability of these organelles.
Studies involving cell culture, animal models, and patients incorporating in vitro and in vivo experimentation have illustrated that abnormalities within the mitochondrial structure and function can relate to neurodegeneration. In turn, this results in motor and cognitive deficits in neuroinflammatory (NI) and neurodegenerative (ND) diseases.
These diseases can include multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (ALS). A commonality between them is that bioenergetic deficit results from mitochondrial dysfunction.
Impaired functionality in the mitochondria can have severe consequences such as an increase in ROS production and oxidative stress, further damaging the mitochondria and worsening neurodegenerative progression.
However, exciting research into the dysfunction and recovery of mitochondria has found that this restoration of mitochondria and its associated functionality can uplift clinical symptoms in cellular and animal models of NI and ND diseases.
Such findings illustrate how crucial strategies to restore mitochondrial homeostasis are for developing promising therapies for NI and ND diseases. These therapeutic developments should be specific for intracellular targets in the central nervous system (CNS) to mitigate systemic side effects experienced by patients diagnosed with these diseases.
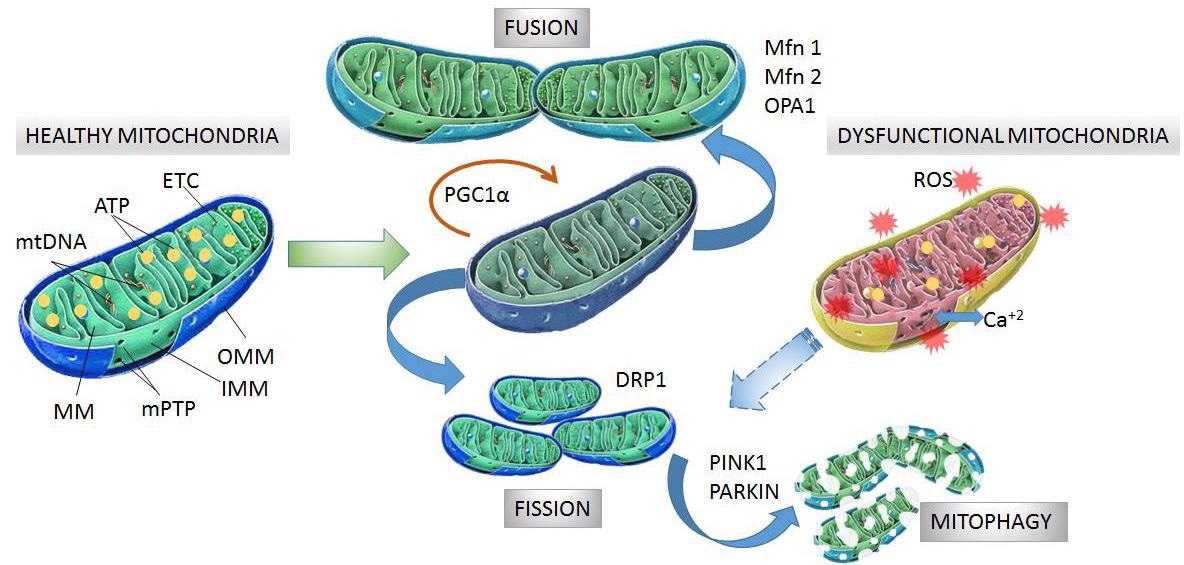
Structure and processes involved in dynamics of healthy and dysfunctional mitochondria. Healthy mitochondria display coordinated and dynamic processes of fusion and fission in order to regulate their morphology, size, and number. After mitochondrial biogenesis guided by PGC-1α protein, fusion generates an interconnected mitochondrial network, which is orchestrated by OPA1, Mfn1 and Mfn2 proteins. Fission results in small size mitochondria without mtDNA replication due to fragmentation and separation from the mitochondrial network, which is a process driven by dynamin- related protein (DRP1). Fragmented mitochondria are degraded by mitophagy, which is a process involving PINK1 and PARKIN proteins. Dysfunctional mitochondria showing alterations in structure and function in neurodegeneration are degraded by mitophagy. Mitochondrial dynamics are maintained by constant activity and precise balance between the biogenesis and clearance of fragmented and defective organelles. mtDNA: mitochondrial DNA, ATP: adenosine triphosphate, ETC: electron transport chain, MM: mitochondrial matrix, mPTP: permeability transition pore, OMM: outer mitochondrial membrane, IMM: inner mitochondrial membrane, PGC-1α: peroxisome proliferator activated receptor-gamma coactivator 1-alpha, Mfn1 and Mfn2: mitofusins 1 and 2, OPA1: optical atrophy 1 protein, DRP1: dynamin-related protein, PINK1: PTEN-induced kinase 1, PARKIN: Parkin RBR E3 ubiquitin-protein ligase. Image Credit: González, L., Bevilacqua, L. and Naves, R.
Novel Strategies Targeting Mitochondria for NI and ND Diseases
Therapeutic efficacy can be reduced when aiming for intercellular targets due to pharmaceutical formulations and biological barriers; however, this is a limitation for mitochondrial-targeted drug delivery.
The obstacle of targeting mitochondria specifically consists of uptake within recipient cells, endosomal escape, lysosomal degradation, and cytoplasmic retention.
Still, with exciting advancements in nanotechnology, these challenges can be overcome with the introduction of nanocarriers or nanoformulations, which can have a slow and sustained release of potential drugs to reduce the dose and frequency of administrations.
This results in an enhancement of drugs within the target tissue and the mitochondria without impacting healthy tissue, reducing adverse effects.
Another therapeutic strategy to target mitochondrial dysfunction includes targeting and reducing the ROS levels produced in the mitochondria via molecules with antioxidant activity, which could aid with neurodegeneration.
Specific mitochondria-targeted nanosystems that contain antioxidants could have the potential to target oxidative stress and reduce the progression of NI and ND diseases.
This review discusses these significant strategies with mention of cerium oxide (CeO2) nanoparticles that can scavenge superoxide anions, hydrogen peroxide, and peroxynitrite, through being internalized by neurons and accumulate at the outer mitochondrial membrane.
The CeO2 nanoparticles reduce levels of reactive nitrogen species, Aβ-induced mitochondrial fragmentation, and neuronal cell death in a cellular model of Alzheimer’s disease.
The Use of Nanotechnology for Functionalization and Future Therapies
Advancements in nanotechnology have enabled the ability to modify and functionalize nanoparticles and materials that have a higher level of targeting.
Nanostructures can be coated with targeting ligands that can directly target the surface of mitochondria; their nanoscale size ensures that biological barriers are not an obstacle.
Specific drug delivery to the mitochondria can be achieved through triphenylphosphonium (TPP), a lipophilic cation widely studied as a mitochondria-targeted agent. Utilizing this component with antioxidants can be significant for mitochondrial targeting.
TPP-linked antioxidants based on natural hydroxycinnamic derivatives have been illustrated as having preferential mitochondrial localization, enhanced protection against oxidative damage, and mitochondrial defects compared to free molecules both in cellular and animal models as well as in ex vivo assay with samples from NI and ND disease patients.
The utilization of nanotechnology for drug delivery systems for targeting mitochondria organelles can be a promising therapy for NI and ND diseases, which could be low-costs and high in efficacy.
With the direct and indirect role that mitochondria organelles play in these diseases, the aim to recover and protect their structure and functionality would be a potentially significant route to providing an advanced treatment which do not yet have a cure.
Continue reading: Progressing Pulmonary Drug Delivery with Nanoparticles.
Reference
González, L., Bevilacqua, L. and Naves, R., (2021) Nanotechnology-Based Drug Delivery Strategies to Repair the Mitochondrial Function in Neuroinflammatory and Neurodegenerative Diseases. Pharmaceutics, 13(12), p.2055. Available at: https://www.mdpi.com/1999-4923/13/12/2055
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.