Two-dimensional (2D) materials are extremely thin. Usually, just an atom thick, 2D materials display extremely desirable properties for advanced technologies, such as superconductivity, flexibility, etc.
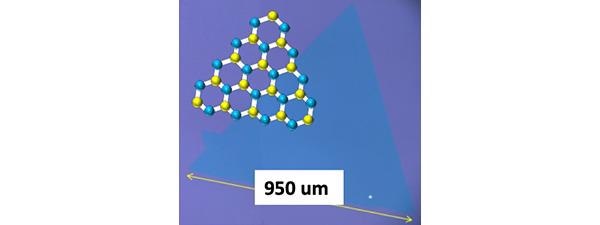 Typical monolayer and single-crystal WS2 grown by a novel monitoring and analysis method. (Image Credit: ©Toshiaki Kato).
Typical monolayer and single-crystal WS2 grown by a novel monitoring and analysis method. (Image Credit: ©Toshiaki Kato).
Developed from carefully transitioning separate components from vapor or gas to crystalline solids, such materials and the processes by which they become infused with such features are still veiled in mystery.
At present, through a unique monitoring and analysis technique, scientists led by Toshiaki Kato at Tohoku University have demonstrated a critical process in the creation of 2D monolayer transition metal dichalcogenide (TMD). They published their method and results in the November 15th issue of the journal Scientific Reports.
"TMD are among the most well-known layered materials," said study author Toshiaki Kato, associate professor in the Department of Electronic Engineering at Tohoku University, observing that large single layers of the material are supported by the incorporation of salts. "Improving the quality of TMD is necessary for realizing future flexible and transparent electrical devices, such as sensor, solar cells, and light emitters."
TMD is created by vaporizing a metal oxide powder and incorporating salts. Conventional methods maintain high temperatures, pushing the molecules of the metal oxide salt vapor to rearrange straight into a crystalline solid. This molecule rearrangement is called nucleation, and it develops into the monolayer TMD.
However, reducing the melting and boiling points of the metal oxide improves this changeover by permitting the vaporized molecules to supersaturate their environment and create a liquid phase before turning into a solid.
"Supersaturation of metal oxide in the vapor phase promotes the creation of liquid-phase precursors, known as the precursor puddle, which promotes vapor-liquid-solid growth over conventional vapor-solid growth," Kato said, observing that the growth rate of vapor-liquid-solid TMD is a minimum of two orders of magnitude higher than that of vapor-solid TMD.
Despite this progress, the critical dynamics of the nucleation phase has not yet been elucidated for salt-assisted growth; achieving this is crucial for both fundamental and industrial applications.
Toshiaki Kato, Study Author and Associate Professor, Department of Electronic Engineering, Tohoku University
To better comprehend the nucleation of vapor-liquid-solid TMD, the scientists set up an imaging monitoring system to monitor how the vapor chemicals are deposited as a solid in TMD synthesis.
In this study, we realized the direct visualization of the phase transition from liquid precursors to solid TMD by monitoring the chemical vapor deposition and automated image analysis. Through this approach, we found a novel nucleation mechanism.
Toshiaki Kato, Study Author and Associate Professor, Department of Electronic Engineering, Tohoku University
In vapor-solid growth, the molecules of the vapor reorient straight into the solid. The scientists discovered that, in vapor-liquid-solid growth, the molecules undergo a two-step nucleation mechanism: The vapor phases into liquid droplets, which develop into stable but variable clusters. As the temperature alters, the molecule clusters develop the crystalline solids.
Such detailed understanding of the TMD nucleation dynamics can be useful for achieving prefect structure control of TMDs, which would be useful for future industrial applications. Our invented method of monitoring chemical vapor deposition and automated image analysis could also be applied to other nanomaterials to more deeply understand their nucleation and growth mechanisms.
Toshiaki Kato, Study Author and Associate Professor, Department of Electronic Engineering, Tohoku University
Going forward, the scientists plan to manipulate the newly exposed nucleation mechanism to create ultra-high quality TMD.
Journal Reference:
Qiang, X., et al. (2021) Non-classical nucleation in vapor-liquid-solid growth of monolayer WS2 revealed by in-situ monitoring chemical vapor deposition. Scientific Reports. doi.org/10.1038/s41598-021-01666-9.