A study published in the journal Materials reported that Nanocrystals of Au1/2Ag1/2CN form in diamond shape epitaxially on pure graphene substrates.

Study: Epitaxial Growth of Diamond-Shaped Au1/2Ag1/2CN Nanocrystals on Graphene. Image Credit: Sebestyen Balint/Shutterstock.com
The nanostructures created by a basic drop-casting procedure exhibit crystallographic alignment to the substrate graphene's lattice framework.
The study gives a new example of one-dimensional cyanide chain (CN) families that produce organized crystal nanostructures epitaxially oriented on two-dimensional substrates and highlights the fundamental physical properties of this largely unexplored nanotechnology.
The Difficulty with Epitaxial Synthesis
The epitaxial production of synthetic nanomaterials on pure faces of two-dimensional substrates has piqued the curiosity of researchers working on nanotechnology and nanocomposites like Van der Waals heterostructures.
Nevertheless, since pure two-dimensional substrate faces are chemically stable and unreactive, it is challenging to form and orient synthetic nanostructured materials right onto pure two-dimensional substrates.
The bulk of prior approaches relied on immobilized free radicals of 2D substrates and intermediary seed elements, resulting in only arbitrarily aligned or imperfectly oriented synthetic nanomaterials on 2D substrates. Otherwise, well heated vapor-phase deposition is necessary to create organized synthetic nanoscale structures directly onto pure 2D substrates. On the basis of past findings, many experts believe that epitaxial growth is very challenging, if not impossible, under ambient circumstances using an aqueous-phase chemical interaction.
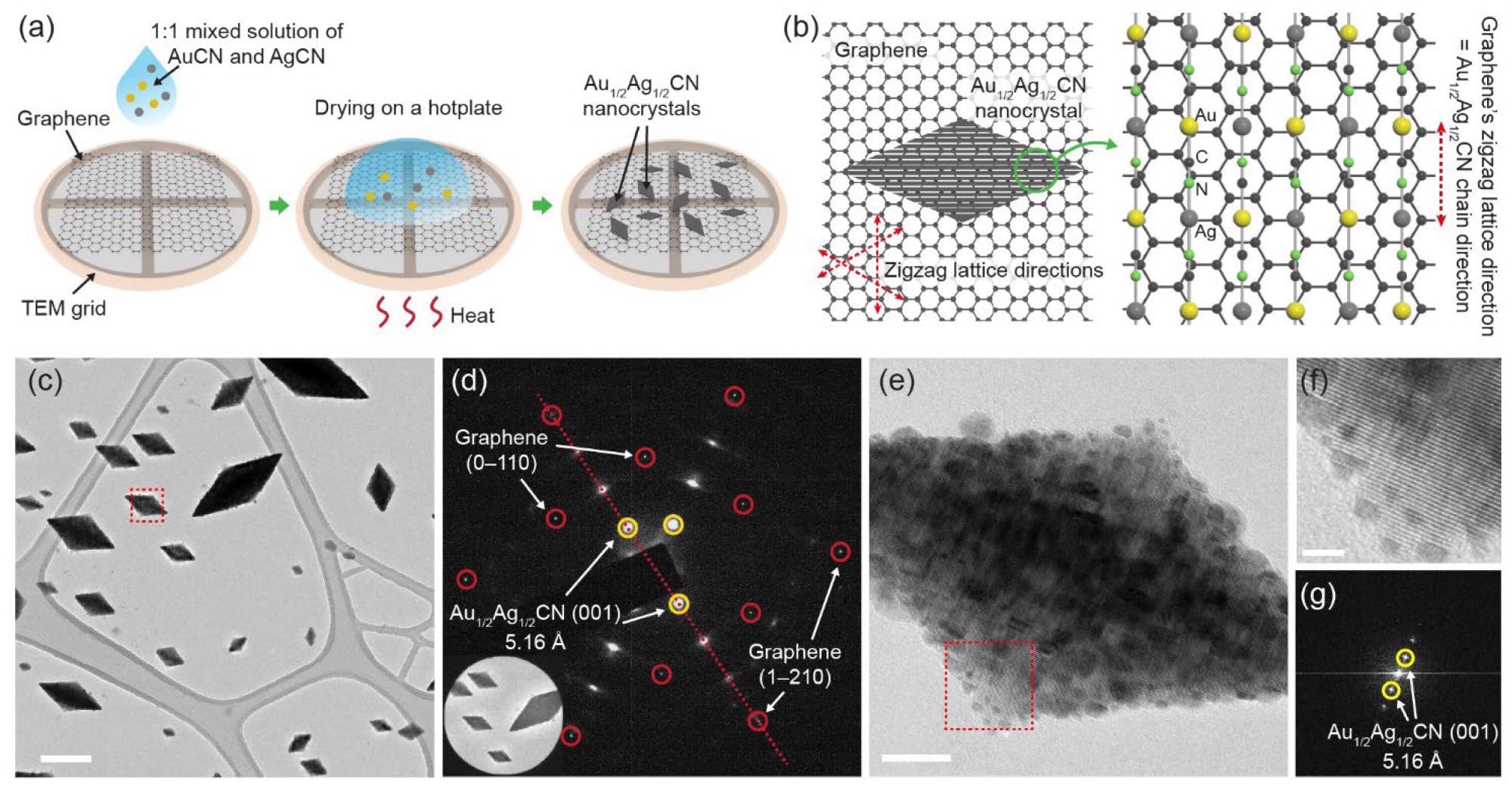
Diamond-shaped Au1/2Ag1/2CN nanocrystals epitaxially grown on single-layered graphene: (a) Nanocrystal synthesis method based on drop-casting. The sample from which (c–g) are obtained is prepared by dropping 0.8 mM Au1/2Ag1/2CN aqueous solution onto CVD-synthesized graphene and drying it at 80 °C; (b) schematic view showing the epitaxial alignment between Au1/2Ag1/2CN and graphene. Lattice spacings of the two materials are presented in Figure S1; (c) TEM image of the diamond-shaped Au1/2Ag1/2CN nanocrystals synthesized on graphene. Scale bar: 200 nm; (d) selected area electron diffraction (SAED) pattern of the nanocrystal-graphene sample. The inset shows an area in (c) where the SAED pattern is measured; (e) TEM image of an individual Au1/2Ag1/2CN nanocrystal (enlarged view of box in (c)). Scale bar: 20 nm; (f) enlarged view of box in (e). Scale bar: 5 nm; (g) fast Fourier transformed (FFT) image of (e). Image Credit: Park, C., Ham, J., Heo, Y., & Lee, W.
Metal Cyanides Present a Workaround
Following the discovery in 2015 that gold cyanides (AuCN) can be epitaxially synthesized on graphene substrate via an aqueous-phase process under ambient conditions, metallic cyanides are likely to represent a unique anomaly in the reactions between synthetic molecules and pure two-dimensional substrates.
Subsequent research found that gold, silver, and copper cyanides and Cu0.5Au0.5CN are epitaxially oriented upon a variety of 2D substrates, such as graphene, boron nitride (h-BN), and molybdenum disulfide (MoS2). Since the one-dimensional chain molecular makeups permit Van der Waals epitaxy and alignment-dependent interactivity to hexagonal crystal lattices, they are crucial for the preferential growth and epitaxial orientation of such metallic cyanides on 2D substrates.
These findings show that the mechanism of preference-based and epitaxial formation may be a generic property of metallic cyanides with one-dimensional chain architectures. While there are many different kinds of cyanide structures on the basis of single and mixed metallic components (Au, Cu, Ag, Fe, and Hg), only the ones cited above have been studied for epitaxial growth.
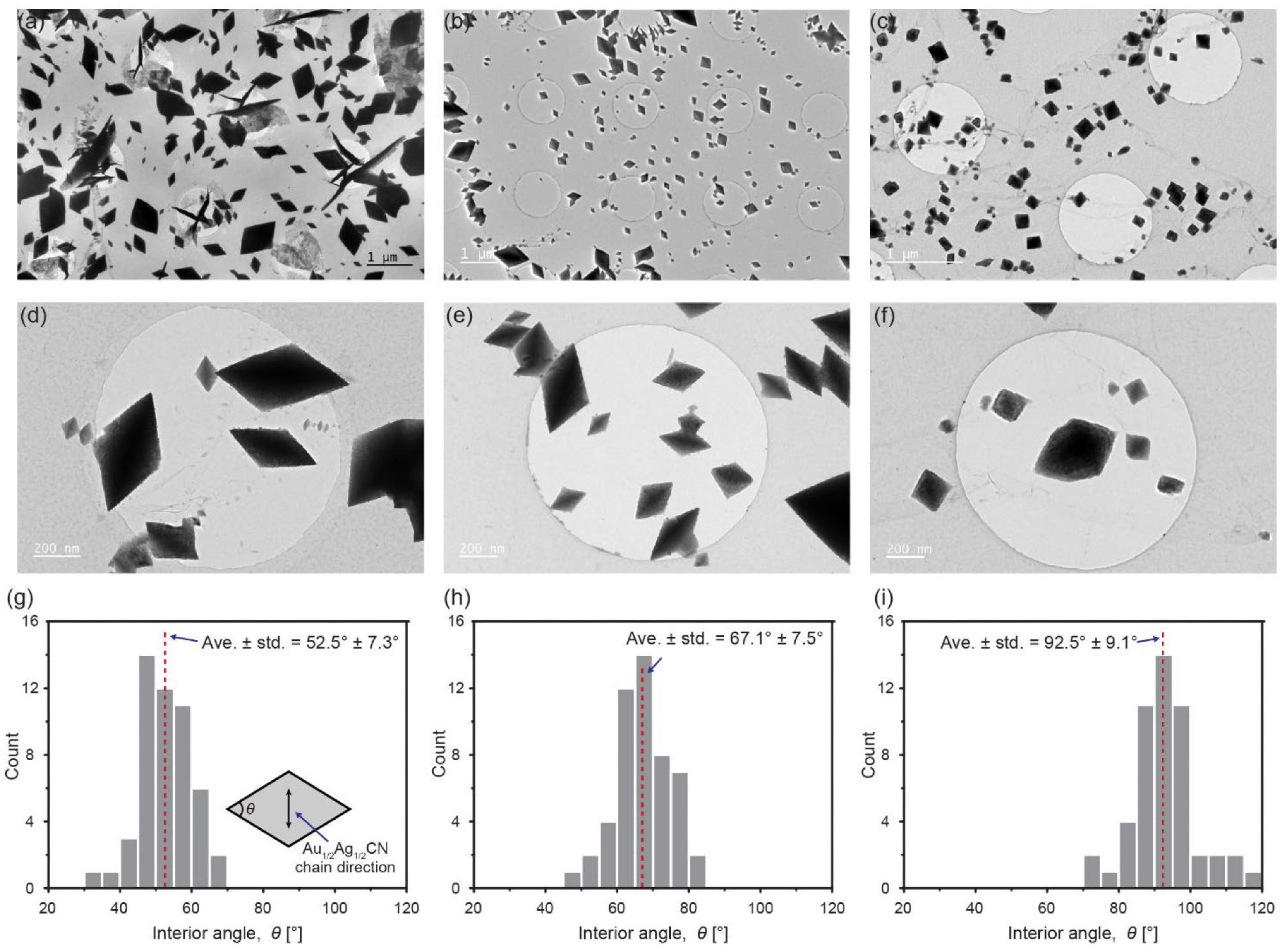
Diamond-shaped Au1/2Ag1/2CN nanocrystals synthesized under different conditions (Au1/2Ag1/2CN concentrations and drying temperatures): (a,d,g) 0.8 mM and 80 °C; (b,e,h) 0.8 mM and 150 °C; (c,f,i) 0.08 mM and 80 °C; (a–f) TEM images of the synthesized Au1/2Ag1/2CN nanocrystals synthesized on single-layered graphene; (g–i) histograms of interior angles of the Au1/2Ag1/2CN nanocrystals in a–f. The inset in g indicates the measured interior angle in an Au1/2Ag1/2CN rhombus (diamond). Image Credit: Park, C., Ham, J., Heo, Y., & Lee, W.
How does Au1/2Ag1/2CN Grow Preferentially on Inert Graphene Surfaces?
The alignment of one-dimensional networks in Au1/2Ag1/2CN nanostructures is parallel to a smaller diagonal of the nanoscale crystalline rhombus. Aligned gold and silver atoms form crystalline lattice threads orthogonal to the one-dimensional network direction and aligned with the rhombus's larger diagonal.
Among the intriguing aspects of the Au1/2Ag1/2CN nanostructures is their preferential formation orientation which is tied to the base graphene lattice frameworks.
Van der Waals epitaxy, which is the heterogeneous nucleation interaction between two components with no immobilized free radicals, may explain this phenomenon. The atoms in an Au1/2Ag1/2CN chain structure are kept together by Van der Waals forces, whereas the networks are bound together by powerful covalent bonding.
As a result, the Au1/2Ag1/2CN crystal may be readily split along the chains with no immobilized free radicals on its surface. Au1/2Ag1/2CN nanostructured crystals can grow selectively on graphene because this surface can produce Van der Waals epitaxial interactivity with pure graphene substrates.
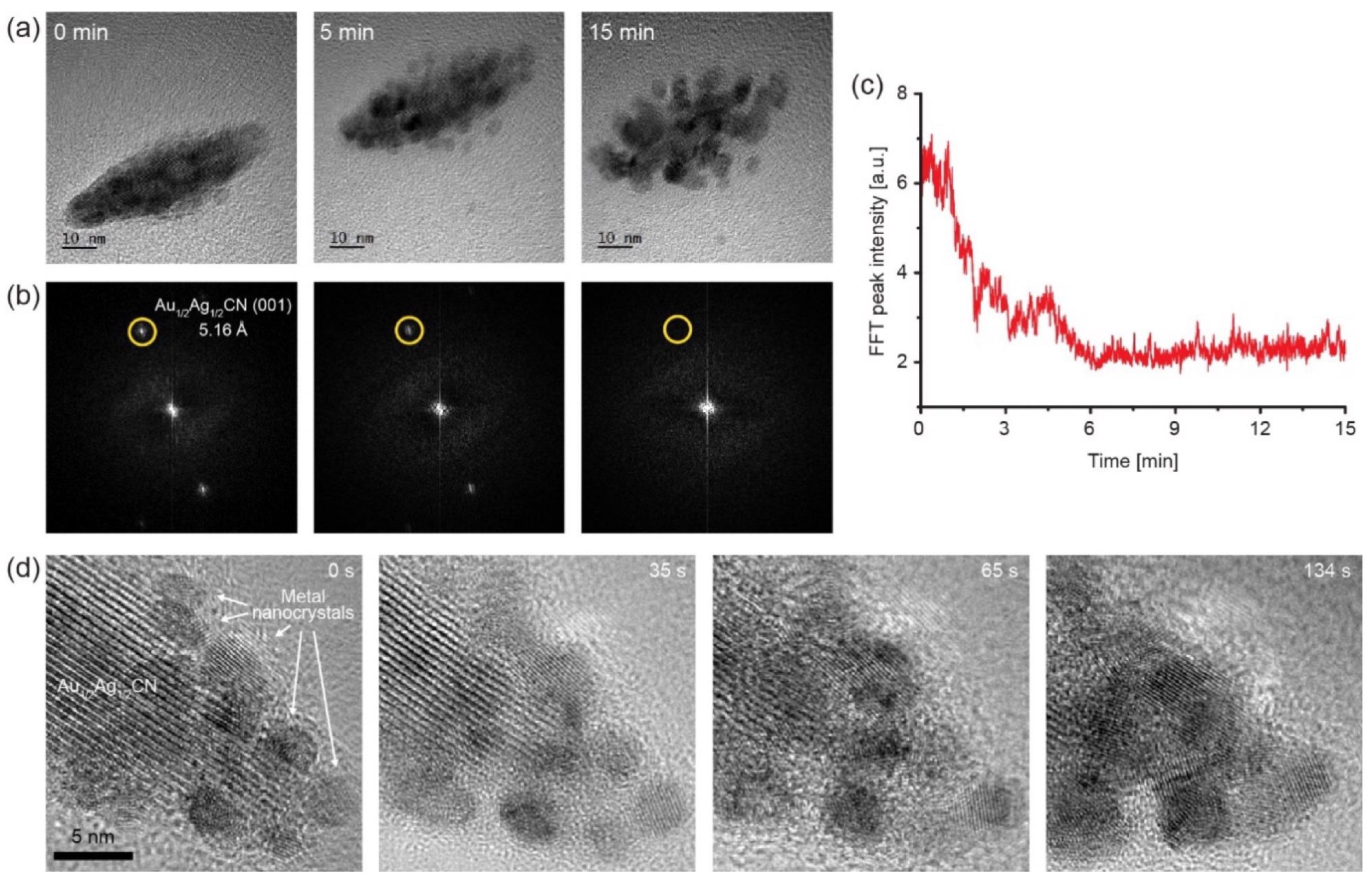
Decomposition process of the Au1/2Ag1/2CN nanocrystal to Au/Ag alloy nanoparticles under electron-beam irradiation: (a) A series of TEM images of the Au1/2Ag1/2CN nanocrystal from Supplementary Video S1; (b) Fourier transformed images of the TEM images in (a); (c) intensity of the Au1/2Ag1/2CN (001) peaks (yellow-circled dots) in (b) over time; (d) a series of TEM images from Supplementary Video S2. Lattice patterns of growing metal nanocrystals can be observed during the in situ TEM imaging. Image Credit: Park, C., Ham, J., Heo, Y., & Lee, W.
Research Findings
On graphene, Au1/2Ag1/2CN forms nanocrystalline structures that are shaped like diamonds. The likelihood that the reported nanocrystals are gold cyanides or silver cyanides is extremely low for the reasons listed ahead.
First, the recorded crystal lattice gaps differ from the major gold or silver cyanide lattice gaps. Second, the rhombic shape appears to be distinctive in that both gold and silver cyanides produce laterally developed nanoscale or microscale wires on two-dimensional substrates such as graphene.
Third, utilizing energy-dispersive X-ray spectroscopy (EDX), it was revealed that the produced nanostructures include both gold and silver components.
The epitaxial development of diamond-shaped Au1/2Ag1/2CN crystalline nanostructures on pure graphene substrates was reported in this study.
The nanostructures formed by basic drop-casting exhibit crystallographic alignment with the basal graphene lattice networks. Akin to other metallic cyanides, this epitaxial orientation emerges from the potential energy landscape of graphene's hexagonal crystal structures, and experimental studies on three-dimensional structures and synthesis requirements suggest that the rhombic 2D configurations emerge from varied formation rates based on positions along and orthogonal to 1D Au1/2Ag1/2CN chains.
Furthermore, upon electron beam irradiation, in situ TEM evaluations indicate the breakdown process of Au1/2Ag1/2CN nanostructured crystals to gold and silver alloy nanocrystals. These findings present yet another instance of one-dimensional cyanide chain groups that produce organized nanocrystals epitaxially oriented on 2D substrates and show fundamental physical properties of the seldom studied Au1/2Ag1/2CN nanocrystals.
Continue reading: Developing a Universal Route to Controlled Nanocrystal Synthesis.
Reference
Park, C., Ham, J., Heo, Y., & Lee, W. (2021). Epitaxial Growth of Diamond-Shaped Au1/2Ag1/2CN Nanocrystals on Graphene. Materials, 14(24). Available at:https://www.mdpi.com/1996-1944/14/24/7569
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.