The combination of transition metal oxides and carbon-based nanomaterials such as spinel NiFe2O4 and 2D graphene oxide for the catalysis of oxygen evolution reaction is the subject of a recent study published in the journal Transactions of Tianjin University.

Study: Comparative Electrocatalytic Oxygen Evolution Reaction Studies of Spinel NiFe2O4 and Its Nanocarbon Hybrids. Image Credit: peterschreiber.media/Shutterstock.com
One of the most important processes for transforming sustainable power into biochemical fuel in the form of hydrogen is the electrocatalytic oxygen evolution reaction (OER). However, the development of suitable and low-cost OER electrocatalysts with high efficacy and substantial longevity is still a major problem.
A promising method for developing advanced catalysts is the fusion of transition metals with activated carbon nanomaterials such as NiFe2O4 and graphene oxide. NiFe2O4's strong performance with 2D graphene oxide is due to its distinctive shape, higher exposed active regions, and high porosity with a large surface area.
Electrochemical Water Splitting Reaction
The world's energy consumption is expanding quickly, with fossil fuel supplies unable to meet the predicted future needs. Additionally, widespread usage of fossil fuels has a detrimental effect on the ecosystem and the wellness of living organisms.
As a result of these issues, substantial work has been carried out globally to develop clean, easy-to-produce, and low-cost renewable energy technology. One of the most intriguing power generation processes is the electrochemical water splitting process, which consists of two half-reactions: hydrogen evolution reaction (HER) on the cathode and oxygen evolution reaction (OER) on the anode. This approach offers several benefits, including good efficiency, low toxicity, and eco-friendliness.
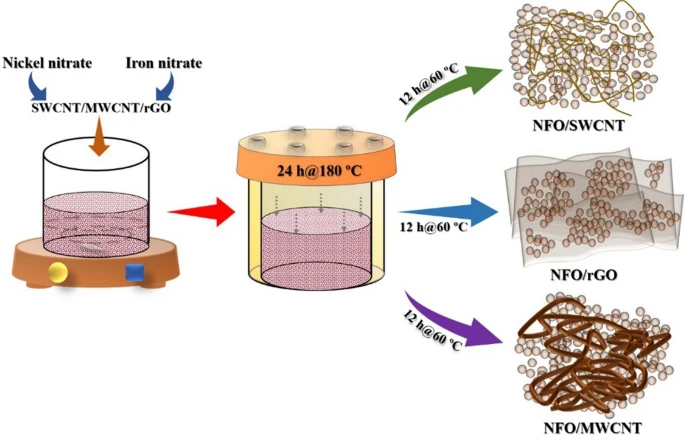
Schematic of the synthesis process of NFO/rGO, NFO/SWCNT, and NFO/MWCNT. © Shinde, P. V. et al. (2021)
Electrocatalysis of Oxygen Evolution Reaction (OER)
Although water electrolysis is a promising method for clean energy generation and conversion, it suffers from the slow reaction rates of OER, requiring a potential greater than the thermal water oxidation potential to accomplish oxygen evolution. Therefore, the production of electrocatalysts with superior performance is very important.
Until recently, the most advanced catalysts have been produced using valuable ruthenium and iridium materials. However, due to their high cost and scarcity on the planet, their widespread usage is significantly hampered. Additionally, their stability is a significant disadvantage for potential implementation.
Major scientific work has been undertaken to develop robust OER catalysts that are inexpensive, plentiful on the planet, and capable of meeting the electrolytic criteria of the water-splitting process.
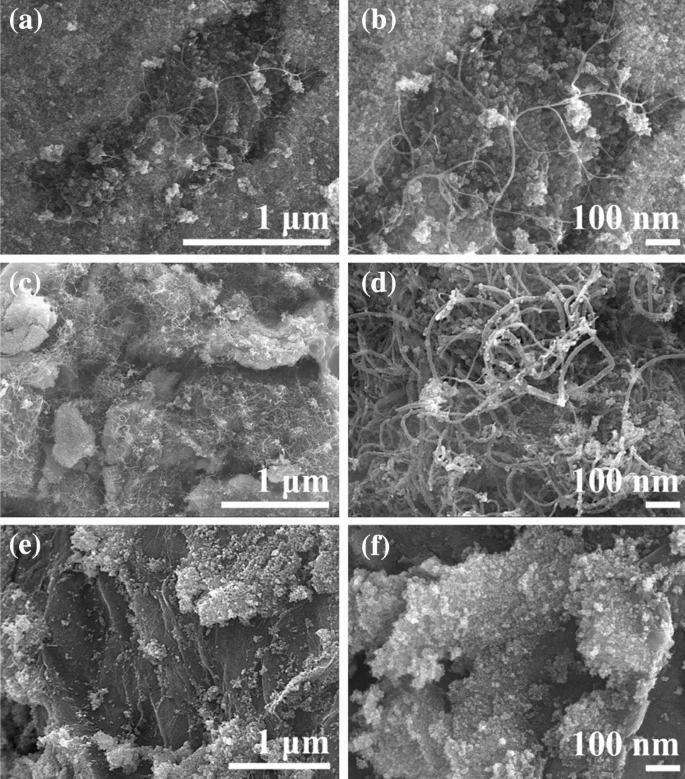
Low- and high-resolution FESEM images of a, b NFO/SWCNT, c, d NFO/MWCNT, and e, f NFO/rGO. © Shinde, P. V. et al. (2021)
Hybrid Materials as Catalysts for OER
Due to their cheap cost, plentiful supply, and nontoxicity, transition metal oxides are a prospective class of materials for the OER process. They also possess intriguing electrolytic and chemical capabilities. Recently, transitional metal-based spinel-type oxides, such as NiFe2O4, have surfaced as efficient electrode materials for use in superconductors, battery packs, and sensor systems.
Apart from being inexpensive, carbon nanomaterials are also robust, biodegradable, environmentally benign, and abundant. Graphene, a solitary sheet of graphite, is used in many applications due to its exceptional mechanical, biochemical, electrical, and charge transfer characteristics. Besides the cost advantage of eliminating the use of rare-earth metals, another significant advantage of adopting carbon nanomaterials such as graphene oxide and CNTs in OER applications is their compositional flexibility. They can operate as support for a variety of materials, which improves the material's conductance.
In this study, the production of hybrid materials made of transitional metal oxides (NiFe2O4) and carbon-based nanomaterials (2D graphene oxide and 1D CNTs) is investigated as a potentially advantageous strategy for electrolytic OER applications.
The conductance of activated carbon nanomaterials and their combinatorial impact with metal sites are credited with enhancing the slow kinetic rates of oxygen evolution reactions.
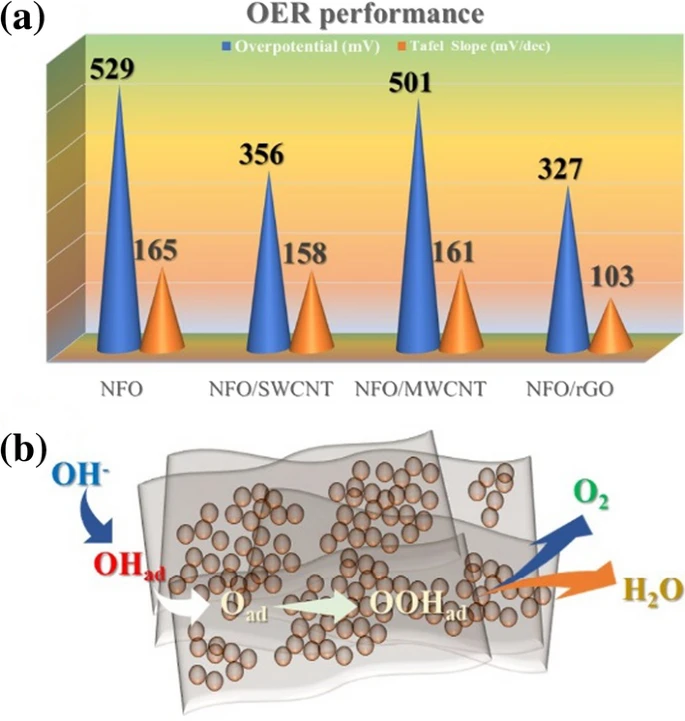
a Comparison of the OER performance of the synthesized catalyst, b schematic of the mechanism of the OER on the catalyst surface.© Shinde, P. V. et al. (2021)
Research Findings
The 2D graphene oxide surface has an abundance of reaction sites for OER, resulting in catalytic performance that is much greater than that of the 1D CNT surface. With a current density of 10 mA/cm2 and a Tafel gradient of 103 mV/dec, the NiFe2O4 with 2D graphene oxide hybrid demonstrates excellent stability and electrolytic performance. This is because of its distinctive shape, more accessible reaction species, and high porosity surface with a large contact area.
In summary, this work explored the impacts of graphene oxide and carbon nanotubes on OER performance through the use of spinel NiFe2O4 hybrids. This hybridization of a metal oxide with an activated carbon substance provides an interesting foundation for the development of an effective electrocatalyst for water splitting reactions.
Continue reading: Game-Changing Electrocatalyst and the Future of Large-Scale Clean Energy.
Reference
Shinde, P. V. et al. (2021) Comparative Electrocatalytic Oxygen Evolution Reaction Studies of Spinel NiFe2O4 and Its Nanocarbon Hybrids. Transactions of Tianjin University. Available at: https://link.springer.com/article/10.1007/s12209-021-00310-x
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.