A novel freeze-dissolving approach has been devised that offers greater efficiency and sustainability compared to the classic freeze-drying process to make superfine powder or nanoparticles.

Study: Freeze-Dissolving Method: A Fast Green Technology for Producing Nanoparticles and Ultrafine Powder. Image Credit: petrmalinak/Shutterstock.com
In the research published in the journal ACS Sustainable Chemistry & Engineering, sphere-shaped ice particles were formed in an aqueous mixture of NH4H2PO4 or NaHCO3 to produce their respective nanoparticles.
What is the Freeze-Drying Method?
Due to their significant specific areas and strong reactivity, nanomaterials and superfine powders are gaining popularity in fields such as sustainable and environmental applications.
Nanoparticles (NPs) and superfine powders are often produced using freeze-drying techniques. The initial stage in the freeze-drying technique is a cryogenic procedure that freezes target particles or molecules in an aqueous mixture.
In the aqueous mixture, water molecules solidify quickly via the fast-freeze stage, generating a framework of crystallized ice. This step is also referred to as ice templating or freeze-casting. The crystallized ice framework forces the targeted dissolved molecules or components to produce a nanoscale scaffolding architecture, which results in substances with nanoscale or microscale pores.
The freezing stage defines the architecture of the scaffolding and the ice template, as well as the crystal architecture of the targeted substances inside the ice templates or scaffolds, based on the freezing settings.
The second phase is a drying procedure that uses the process of sublimation to separate water as ice templates. The ice melts throughout the drying phase, but the targeted substances, particles, or molecules stay within the ice. From inside the ice, freeze-cast NPs or porous substances with identical architecture and characteristics may be retrieved.
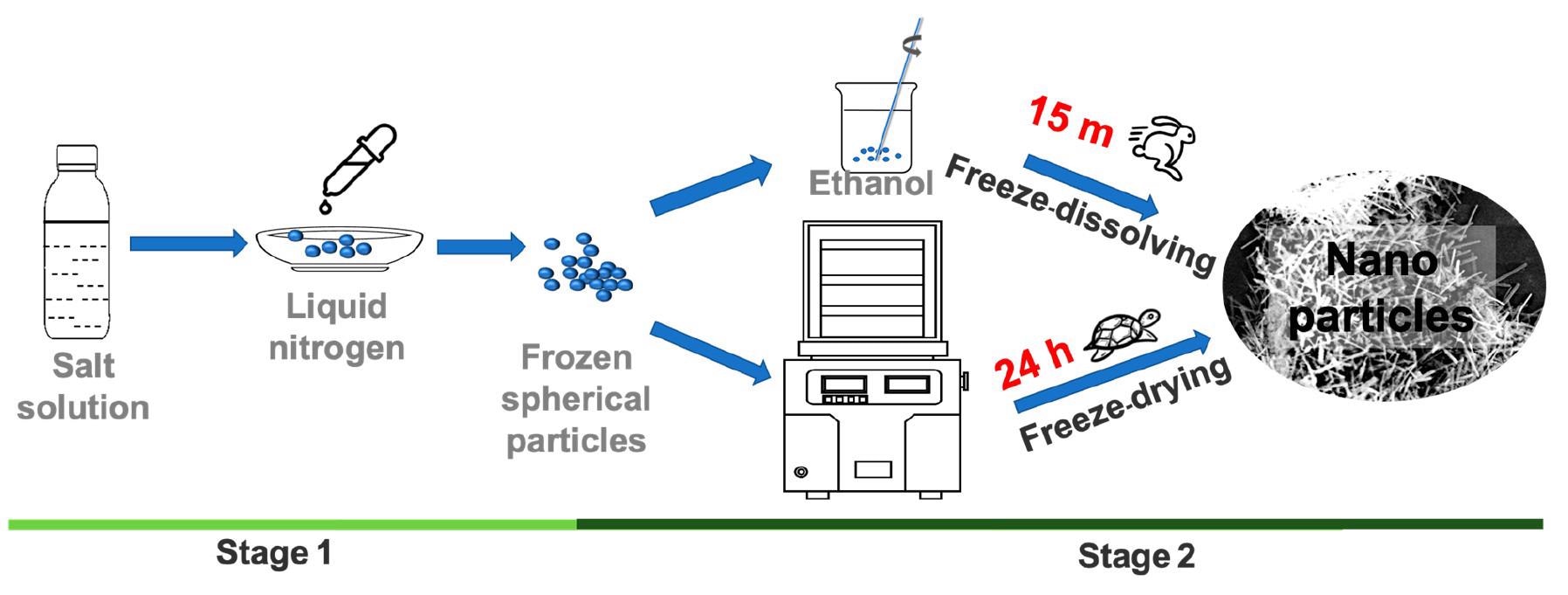
Schematic diagram of experimental setup for the freeze-dissolving method (top) and the freeze-drying method (bottom). © Yu, Q., Wang, Y., Luo, J., & Yang, H. (2022).
Limitations of Freeze-Drying
Due to the cooler temperatures employed in the drying phase, sublimation speeds are sluggish, and batch drying periods for common pharmacological items can take up to multiple days. The production speeds of such batch-based technologies are constrained by poor freeze-drying speeds and extended cycle operation durations.
Some drawbacks may be mitigated by purchasing a bigger freeze-dryer. Unfortunately, it takes much more time to establish perfect vacuum settings, and temperature and pressure are less consistent throughout the container, which could influence output quality. As a result of the cold temperatures and the vacuum arrangement, the drying phase consumes a lot of energy.
How is the Freeze-Dissolving Method Better?
The initial stage in freeze-dissolving is identical to that of freeze-drying, that is, freeze-casting to create ice containing the target components within and build an ice scaffold target architecture.
The ice is then dissolved at a cold temperature, such as a sub-zero temperature in an additional solvent having a low freezing point in the subsequent phase of the freeze-dissolving process. This additional solvent, like ethanol, acts as an antisolvent for the targeted components yet shows miscibility with water.
As a result, the ice scaffold will dissipate fast in the additional solvent, leaving just the targeted components in a solid-state in the mixture, and the architecture of the targeted components produced within the ice will be conserved.
Fire suppression chemicals, baking soda, ammonium dihydrogen phosphate (NH4H2PO4), and sodium bicarbonate (NaHCO3) are water-soluble but do not dissolve in ethanol.
In this work, various quantities of sodium bicarbonate or ammonium dihydrogen phosphate, dissolved in water, were employed to manufacture NPs via the freeze-dissolving technique, which were then evaluated against NPs produced by freeze-drying.
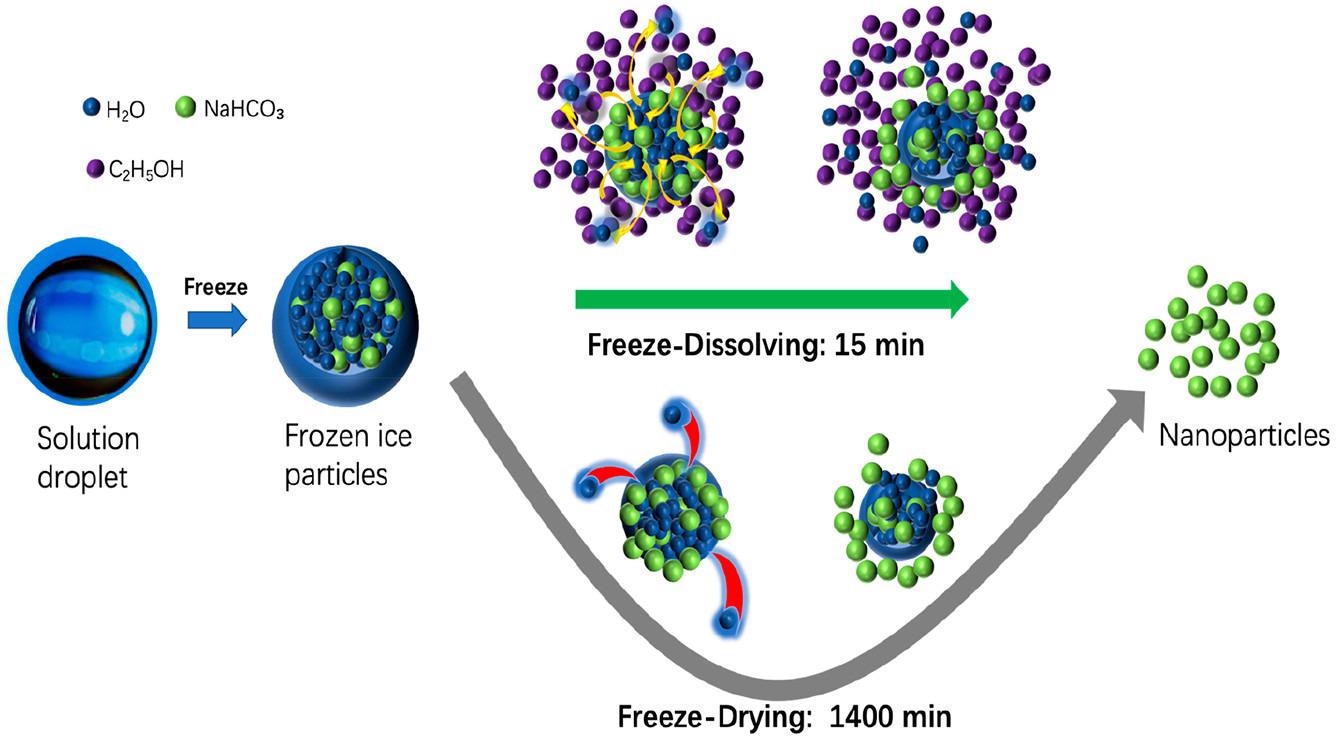
Schematic diagram of the freeze-dissolving and freeze-drying mechanisms for the formation and isolation of NaHCO3 nanoparticles. © Yu, Q., Wang, Y., Luo, J., & Yang, H. (2022).
Important Findings
To extract superfine powder and NPs from ice templates within frozen particles, the proposed freeze-dissolving process offers greater efficiency and sustainability compared to the conventional freeze-drying approach.
Particles of sodium bicarbonate and ammonium dihydrogen phosphate aqueous mixtures were quickly frozen to produce sphere-shaped ice particles, which were then filled with NPs and superfine powder of NaHCO3 or NH4H2PO4.
The frozen components were dispersed in ethanol for 5 minutes at 10 °C using the freeze-dissolving procedure to separate the ice scaffold. The freeze-drying approach, on the other hand, needed 1400 minutes to separate the ice scaffold via the process of sublimation. In identical experimental settings, the dimensions of the end products generated by the freeze-dissolving approach were comparatively small as opposed to those produced by the freeze-drying approach.
The freezing-dissolving approach reported in this study is approximately 100 times quicker and consumes roughly 100 times lesser energy as compared to the freeze-drying approach, without the need for a large facility or a vacuum. As a result, the freeze-dissolving process is likely to be used on an industrial scale with less time, energy, and footprint.
Reference
Yu, Q., Wang, Y., Luo, J., & Yang, H. (2022). Freeze-Dissolving Method: A Fast Green Technology for Producing Nanoparticles and Ultrafine Powder. ACS Sustainable Chemistry & Engineering. Available at: https://doi.org/10.1021/acssuschemeng.2c02270
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.