Nanosized extracellular vesicle (EV) exosomes serve as reliable biomarker sources. However, cancer biomarker identification through exosome multi-omic molecular information mapping is challenging due to heterogeneous populations of exosomes derived from diverse cell types.
Study: Nano pom-poms prepared exosomes enable highly specific cancer biomarker detection. Image Credit: Meletios Verras/Shutterstock.com
In an article published in the journal Communications Biology, researchers introduced novel three-dimensional (3D) nanographene immunomagnetic particles for specific capture and release (defined by marker) of the intact exosome. The designed novel 3D-structured nanographene particles had a unique morphology of flower pom-poms.
Moreover, the capture and release of intact exosomes were via photo-click chemistry. This exosome isolation approach allowed the identification of cancer biomarkers with enhanced sensitivity and specificity. The multi-omic exosome analysis for cancer biomarker identification was performed using tissue fluids obtained from a bladder cancer patient.
The nanographene immunomagnetic particles prepared exosomes showed a distinct in vivo biodistribution, indicating integral quality with high viability. This nanographene immunomagnetic particles-based technique is a facile approach applicable to various biological fluids. Amending the developed method facilitates scale-up, enrichment, and high-throughput exosome isolation.
What are Cancer Biomarkers?
Despite continuous efforts to develop cancer biomarkers, only a few are approved clinically by the United States Food and Drug Administration (USFDA), including progesterone receptor (PR), human epidermal growth factor receptor 2 (HER-2/neu), cancer antigen 125 (CA-125), and prostate-specific antigen (PSA).
EVs are emerging biomarker sources explored to discover a wide range of cancer biomarkers to achieve cancer diagnosis, drug target and delivery, and immunotherapy. Exosome-type small EVs and their molecular components are associated with various physiological functions and disease pathologies. The exosomes are secreted from tumor cells and are enriched with tumor markers. Consequently, their presence increases in plasma and ascites of the patients diagnosed with cancer.
Body fluids contain different types of EVs. Hence, cellular-specific homogenous exosome populations are not attainable. The cell-secreted membrane vesicles (EVs) are heterogeneous in origin, present in multiple subpopulations, in the broad size range of 30 to 1000 nanometers. Thus, due to the heterogenicity in vesicles and their overlapping size ranges, the exact cellular origin of the exosomes cannot be identified to understand the disease pathogenesis.
The existing purification methods utilized to recover extracellular materials (EMs) with vesicle or non-vesicular molecules include ultracentrifugation-based (UC) precipitation and precipitation polymer kits. However, these approaches are not scalable, and the exosome populations originating from different cell types or EV subtypes cannot be differentiated. Studying cancer biomarkers from exosomes derived from cancer cells is therefore challenging.
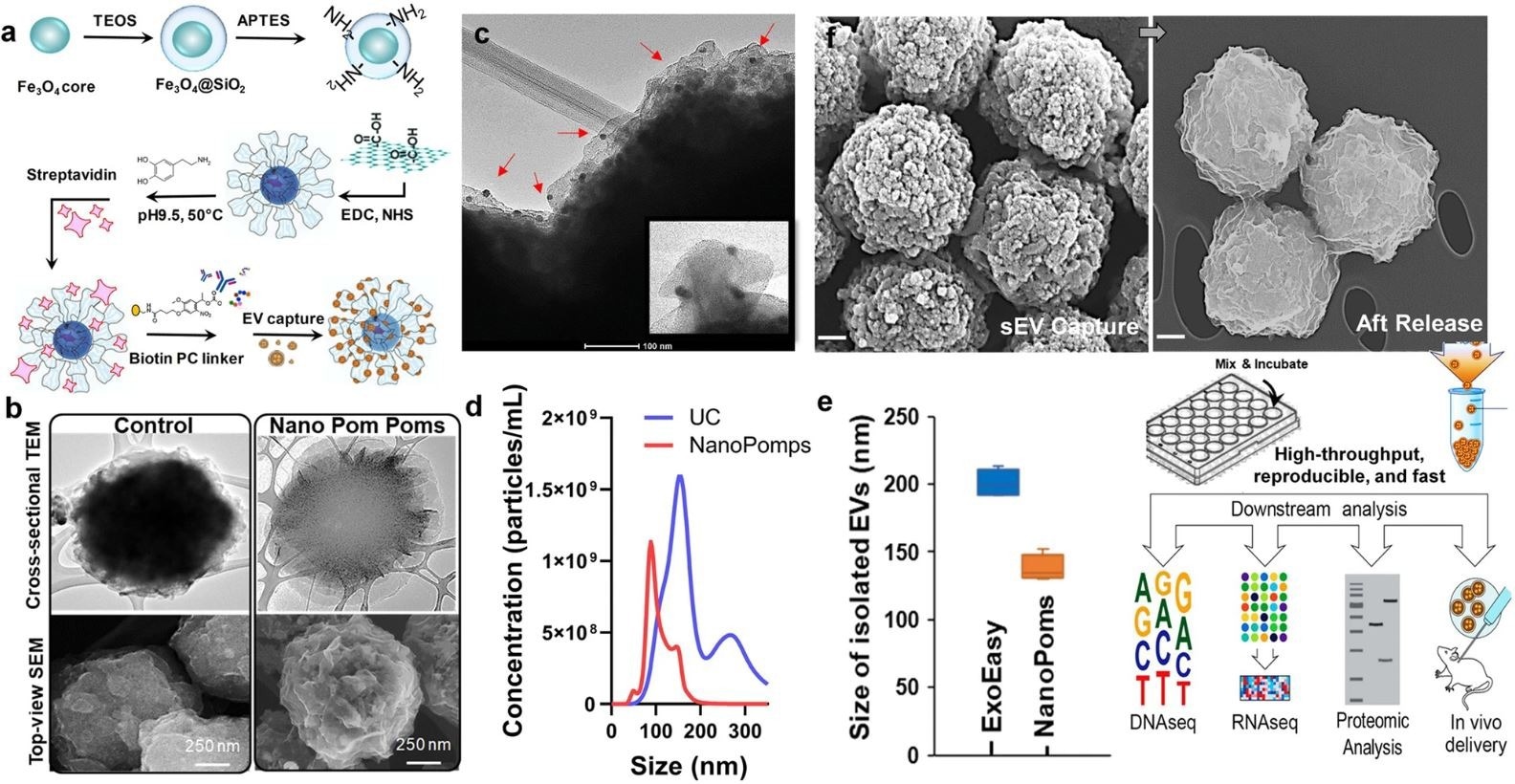
a Schematic illustration of the fabrication of Nano pom poms. b TEM and SEM images showing the unique 3D nano-scale flower pom-poms morphology compared to commercial immunomagnetic beads. c The immune gold nanoparticle staining TEM imaging of captured exosomes fully covering Nano pom-poms surface. Captured EVs are confirmed by antiCD63 gold nanoparticles. The insert shows the captured single exosomes in the size range of ~100 nm with three gold nanoparticles bound (~10 nm). d Nanoparticle tracking analysis of NanoPoms isolated exosomes with much narrower size distribution in comparison with UC isolated EVs. e Nanoparticle tracking analysis of the size of NanoPoms isolated exosomes (n = 4 independent experiments, mean ± SD), compared with ExoEasy isolation (n = 4 independent experiments, mean ± SD), which showed reproducible and smaller size of exosomes from NanoPoms preparation. f SEM images showing the dense exosomes are captured covering the surface of Nano pom-poms, and can be completely released via on-demand photo-cleavage. After release, intact exosomes can be harvested for downstream multi-omic analysis including next generation sequencing of DNAs, RNAs, western blotting and proteomic analysis, as well as in vivo study. © He, N., Thippabhotla, S., Zhong, C., Greenberg, Z., Xu, L., Pessetto, Z., Godwin, AK. et al. (2022)
Nanographene Exosomes Towards Specific Cancer Biomarker Detection
The present study demonstrated a novel approach for specific capture and release of the intact exosomes that utilizes 3D-structured nanographene immunomagnetic particles. These nanographene immunomagnetic particles have a flower pom poms-like morphology, and the exosome capture and release mechanisms were based on photo-click chemistry.
The intact exosomes for the present study were isolated from different biological fluids, including human urine and blood, cow milk, and cell culture medium. Furthermore, the isolation of exosomes via nanographene immunomagnetic particles facilitated the effective identification of cancer biomarkers with high sensitivity and specificity compared to those isolated via immunomagnetic beads.
The in vivo biodistribution was tested for the exosomes released from nanographene immunomagnetic particles. The results showed distinct biodistribution patterns with insignificant alterations in surface properties of the exosomes, implying the potential of nanographene immunomagnetic particles in therapeutic development.
The exosomes derived from bladder cancer tissue fluids (urine and plasma) were compared with those derived from tumor tissues by next-generation sequencing (NGS) of miRNAs, global proteome, and somatic DNA mutations to achieve an invasive and ultra-sensitive diagnosis of bladder cancer.
The results revealed that compared to ultracentrifugation or bead isolation approaches, exosomes isolated from nanographene immunomagnetic particles showed improved sensitivity and specificity toward detecting urological tumor biomarkers.
The impact of external stimuli like the light release was observed on exosome isolation via a nanographene immunomagnetic particles-based approach in which miRNA profiles were compared in the presence and absence of the light release process. The light release process exhibited specificity by only releasing captured exosomes. These observations confirmed the quality and integrity of nanographene immunomagnetic particles-prepared exosomes as a robust and facile method.
What Did the Study Find?
To summarize, the exosome isolation via nanographene immunomagnetic particles-based method was applied to various biological fluids, including human urine and blood, cell culture medium, and cow’s milk. This isolation method was simple and did not require an additional ultracentrifugation process.
The 3D structure of nanographene immunomagnetic particles and the regulated capture-release process by specific markers helped prepare homogenous exosome subpopulations that could enrich the cancer biomarkers.
The NGS and droplet digital polymerase chain reaction (ddPCR) demonstrated that DNA isolation from nanographene immunomagnetic particles-prepared exosomes could be enriched for DNA mutations in bladder cancer-relevant tumors and help in cancer biomarker identification.
Reference
He, N., Thippabhotla, S., Zhong, C., Greenberg, Z., Xu, L., Pessetto, Z., Godwin, AK. et al. (2022) Nano pom-poms prepared exosomes enable highly specific cancer biomarker detection. Communication Biology. https://www.nature.com/articles/s42003-022-03598-0
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.