Reviewed by Mila PereraOct 27 2022
A research group headed by Prof. Jie Zeng of the Chinese Academy of Sciences and international associates devised a novel “nanoglue” technique to maintain atomically scattered metal catalysts in a report published in Nature.
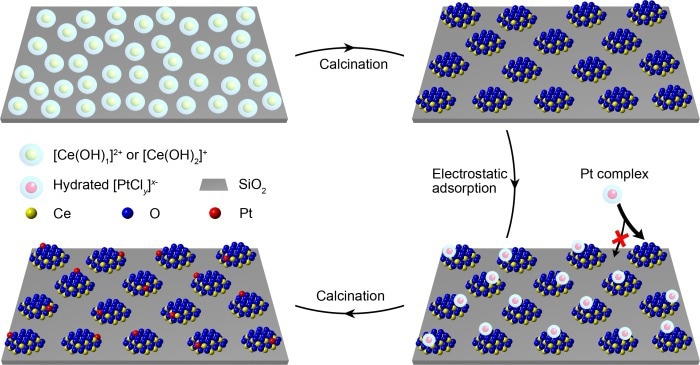
The fabrication processes of functional ceria nanoglue and ceria-supported Pt single-atom catalysts. Image Credit: Prof. Jie Zeng’s team
The atomically dispersed metal catalyst has garnered significant attention in the field of heterogeneous catalysis due to its unique geometric and electronic features, superior atom efficacy, and uniform active sites.
However, due to the high surface energy, the highly distributed metal atoms migrate and agglomerate readily, which results in limited stability. Alternatively, they intensely interact with the support, becoming catalytically passivated. As a result, obtaining “moving but not agglomerating” metal sites that can improve catalytic activity and stability has been a significant challenge in catalysis.
In light of this, the researchers created a new “nanoisland” catalyst (also known as nanoglue) wherein active metal atoms are isolated on “islands” where they can move inside respectively, but migration to neighboring “islands” is inhibited, resulting in “moving but not agglomerating” atom sites.
To accomplish this goal, proper materials for “nanoislands” and supports had to be chosen. Metal atoms’ affinity for “nanoislands” should be substantially stronger than metal atoms’ affinity for support; otherwise, metal atoms can easily form their own “nanoislands.”
As a result, in the constructed model catalyst, the investigators used oxides with high affinity to metal atoms as “islands” (like ceria) and weak-interaction oxides (like silica) as support to stabilize “islands.”
To properly isolate metal atoms, the “islands” on the support had to be small enough in size and sufficiently dense in number. Traditional synthesis processes (such as impregnation) produce huge, non-uniform particles that are unsuitable for use as “islands.”
Researchers devised a robust electrostatic adsorption mechanism in an aqueous solution. The high-density cerium atoms were first deposited on the silica surface, then calcined into isolated "islands" with sizes less than two nanometers.
The next task was to precisely arrange the metal atoms on the “nanoislands” rather than on the support. To that purpose, the researchers applied the strong electrostatic adsorption approach, with a difference in the point of zero charges of ceria and silica, to oppositely charged ceria islands and silica surfaces.
The negatively charged platinum precursor could only be adsorbed on positively charged ceria “nanoislands” instead of negatively charged silica supports, meaning that platinum atoms were produced only on ceria islands. Due to the small size of the ultra-small “nanoislands” and the low concentration of platinum precursor, each “island” received less than one platinum atom on average.
The research demonstrated that platinum atoms atop ceria “nanoislands” could withstand sintering during air calcination up to 600 °C. Platinum atoms, specifically, were shown to travel inside their own “island” in hydrogen-reducing conditions at increased temperatures rather than spreading to neighboring “islands.”
Furthermore, the hydrogen-activated catalyst’s carbon monoxide oxidation activity was boosted by two orders of magnitude, as well as outstanding stability.
This research presents a novel technique for increasing catalytic activity and stability. This “nanoglues” concept is intended to be applied to many catalytic reactions in the future by selecting appropriate supports, “nanoislands,” and active metal atoms.
Yong Wang of Washington State University, Bruce C. Gates of the University of California, Davis, and Jingyue Liu of Arizona State University collaborated on this project.
Journal Reference:
Li, X., et al. (2022) Functional CeOx nanoglues for robust atomically dispersed catalysts. Nature. doi.org/10.1038/s41586-022-05251-6.