.jpg) By Pritam RoyReviewed by Susha Cheriyedath, M.Sc.Nov 15 2022
By Pritam RoyReviewed by Susha Cheriyedath, M.Sc.Nov 15 2022In an article published in Scientific Reports, researchers examined a relatively unknown cerium dioxide (CeO2) nanoparticle’s antiviral potential. The ability of two nano-CeO2 with opposite surface charges, (+) and (-), to reduce the plaque-forming units (PFU) of two non-enveloped and four enveloped viruses following a one-hour exposure was investigated.

Study: Antiviral efficacy of cerium oxide nanoparticles. Image Credit: Inkoly/Shutterstock.com
At 20 milligrams per liter, statistically relevant antiviral activity against the influenza virus and the enveloped coronavirus SARS-CoV-2 was detected. Similarly, antiviral action was demonstrated at 200 milligrams per liter for the other two enveloped viruses, bacteriophage f6 and transmissible gastroenteritis virus (TGEV). As hypothesized, non-enveloped viruses were substantially less sensitive to nano-CeO2.
The MS2 bacteriophage displayed a minor non-monotonic response to a higher concentration of nano-CeO2(-). Contrarily, the encephalomyocarditis (EMCV) picornavirus exhibited no drop in PFU until the highest measured concentration of nano-CeO2(-) was at 2000 milligrams per liter. It was possible to deduce that the antiviral activity of nano-CeO2 was not caused by released Ce-ions or by the nonspecific impact of nano particulates by parallel testing Ce3+ ions and SiO2 nanoparticles.
Additionally, the authors demonstrated that nano-CeO2 had greater antiviral effectiveness than silver (Ag) nanoparticles. This result, together with negligible nano-CeO2’s antibacterial activity and non-existence of cytotoxicity, made CeO2 nanoparticles suitable for antiviral uses.
The Role of Metal Nanoparticles as Novel Antiviral Agents
During the COVID-19 pandemic, there has been a notable increase in the quest for antiviral agents. Antiviral agents are materials that can render viruses inactive, prevent them from infecting their host cells, or restrict their capacity to multiply. It has recently been realized that nanotechnology has the potential to aid in the creation of antiviral therapeutics.
Nanoparticles of metal and metal oxide are proposed as potential antiviral nanomaterials. These metals and their oxides exert their activity through various multimodal action mechanisms, such as direct binding to the virus surface, preventing viral binding from hosting cells, or inhibiting the interaction with the viral genome.
One of the most researched antiviral nanoparticle types is nanosilver. Its possible mechanisms of action include the attachment of nanosilver particles to the viral genetic material and the attachment of nanosilver particles to the viral outer surface, both of which can further impede viral reproduction.
Several viruses, such as herpes simplex, human immunodeficiency virus (HIV), influenza, adenoviruses, noroviruses, SARS-CoV-2, and others, have shown that silver nanoparticles effectively reduce infective viral counts.
In this work, the authors concentrated on CeO2-based nanomaterials since CeO2 was antivirally effective with negligible influence on microbiota or human cells. The goal was to identify the antiviral activities of ultrasmall CeO2 nanoparticles against various non-enveloped and enveloped mammalian viruses and certain bacteriophages. Also, the antiviral activity of Ag and silica (SiO2) nanoparticles was investigated. Ag nanoparticles were typically considered to display strong antiviral activities, while SiO2 was presumed to be biologically neutral.
Additionally, these nanoparticles’ possible surface protein inactivation was investigated by taking SARS-CoV-2 as an example to determine the basis of CeO2 nanoparticles’ antiviral action. Finally, the bactericidal activities of CeO2 and other chosen nanoparticles against Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli and their cytotoxic effects were compared.
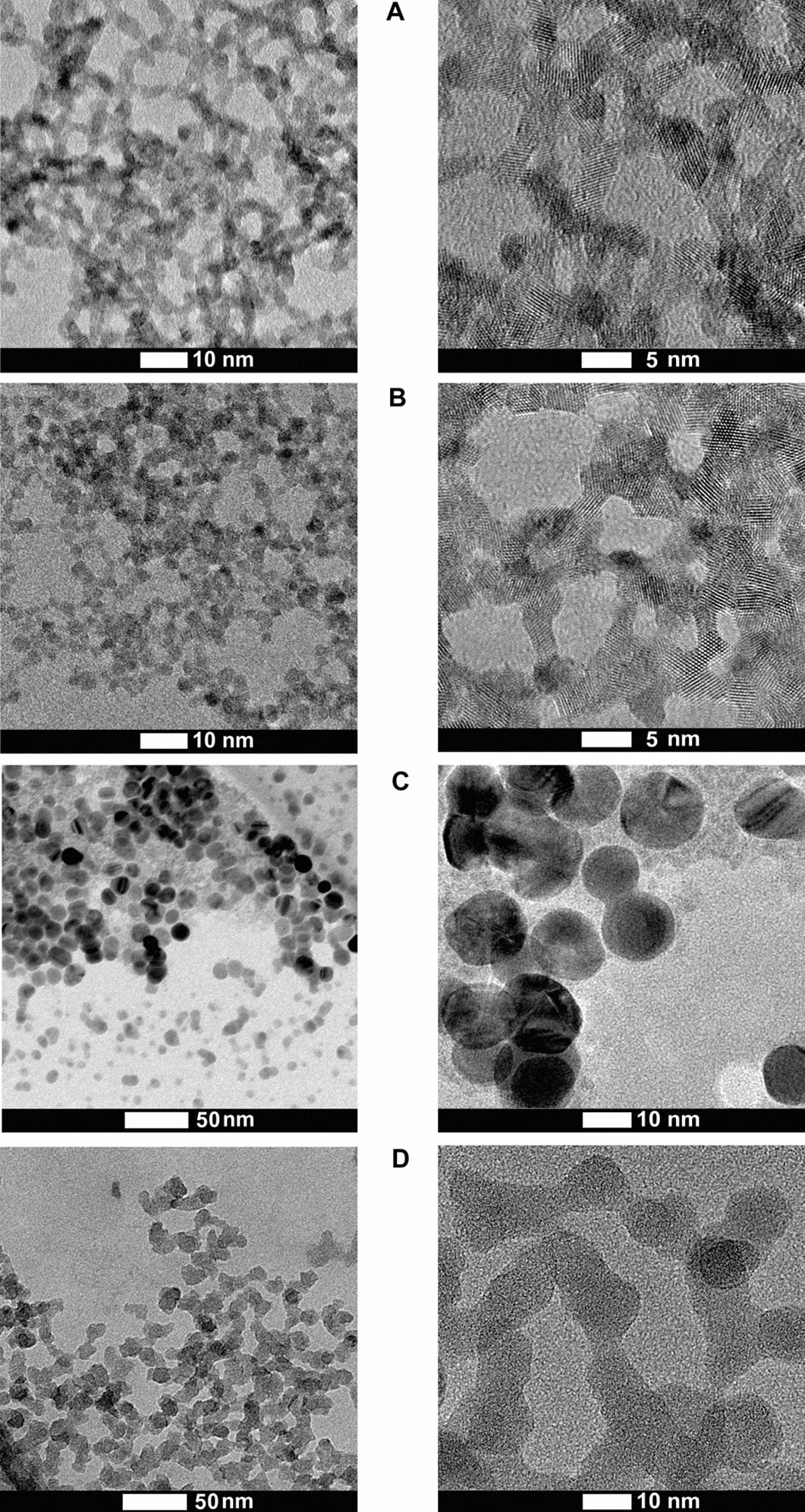
Figure 1. HRTEM images of synthesized nanoparticles. (A) Nano-CeO2(+), (B) nano-CeO2(−), (C) nano-Ag, (D) nano-SiO2. © Nefedova, A., et al. (2022).
Experimental Set-Up
The target materials for this study’s antiviral potential assessment were ceria nanoparticles, produced using two different procedures that made nano-CeO2 with negative and positive surface zeta potential. Also, the antiviral actions of nano-CeO2 were contrasted with those of nano-Ag, which were assumed to have potent antiviral properties (positive control), and nano-SiO2, which were considered biologically inactive (negative control).
A decline in infective counts (expressed as plaque-forming units, PFU) of two bacteriophages and four mammalian viruses were examined after a one-hour exposure to the nanoparticles to demonstrate the antiviral potential of nano-CeO2, nano-Ag, and nano-SiO2. Nano-CeO2 with negative and positive surface zeta potentials were employed to understand how the CeO2 surface affected the antiviral activity of nano-CeO2.
The findings of the antiviral assay demonstrated that nano-CeO2 influenced the infectivity of most bacteriophages and mammalian viruses despite being non-toxic to bacteria and mammalian cells. For SARS-CoV-2, f6, and influenza virus, a significant decline in viral infectivity due to a one-hour nano-CeO2(+) contact was reported starting at 20 milligrams per liter and starting at 200 milligrams per liter for TGEV.
Infectivity for nano-CeO2(+) to 2000 milligrams per liter did not significantly decrease for non-enveloped viruses. Starting at 20 milligrams per liter, nano-CeO2(-) impacted influenza and SARS-CoV-2 virus’ ability to infect. Also, the TGEV and f6 virus’ ability to infect was observed starting at 200 milligrams per liter. Non-enveloped viruses displayed the lowest susceptibility to nano-CeO2(-), following the pattern of nano-CeO2(+).
The antiviral efficiency of nano-CeO2 was compared to nano-Ag, a positive control, and nano-SiO2, a negative control. Naturally, nano-Ag and nano-SiO2 demonstrated no effect on the non-enveloped viruses, which exhibited no sensitivity to nano-CeO2.
Antibacterial activity against Gram-positive S. aureus and Gram-negative E. coli was examined concurrently with antiviral characteristics. The findings showed that the antiviral effects of CeO2 nanoparticles were more evident than their bactericidal efficiency because neither nano-CeO2(+) or nano CeO2(-) to 2000 milligrams per liter reduced the viable counts, reported as colony-forming units, CFU of S. aureus or E. coli by greater than 2 logs (99%).
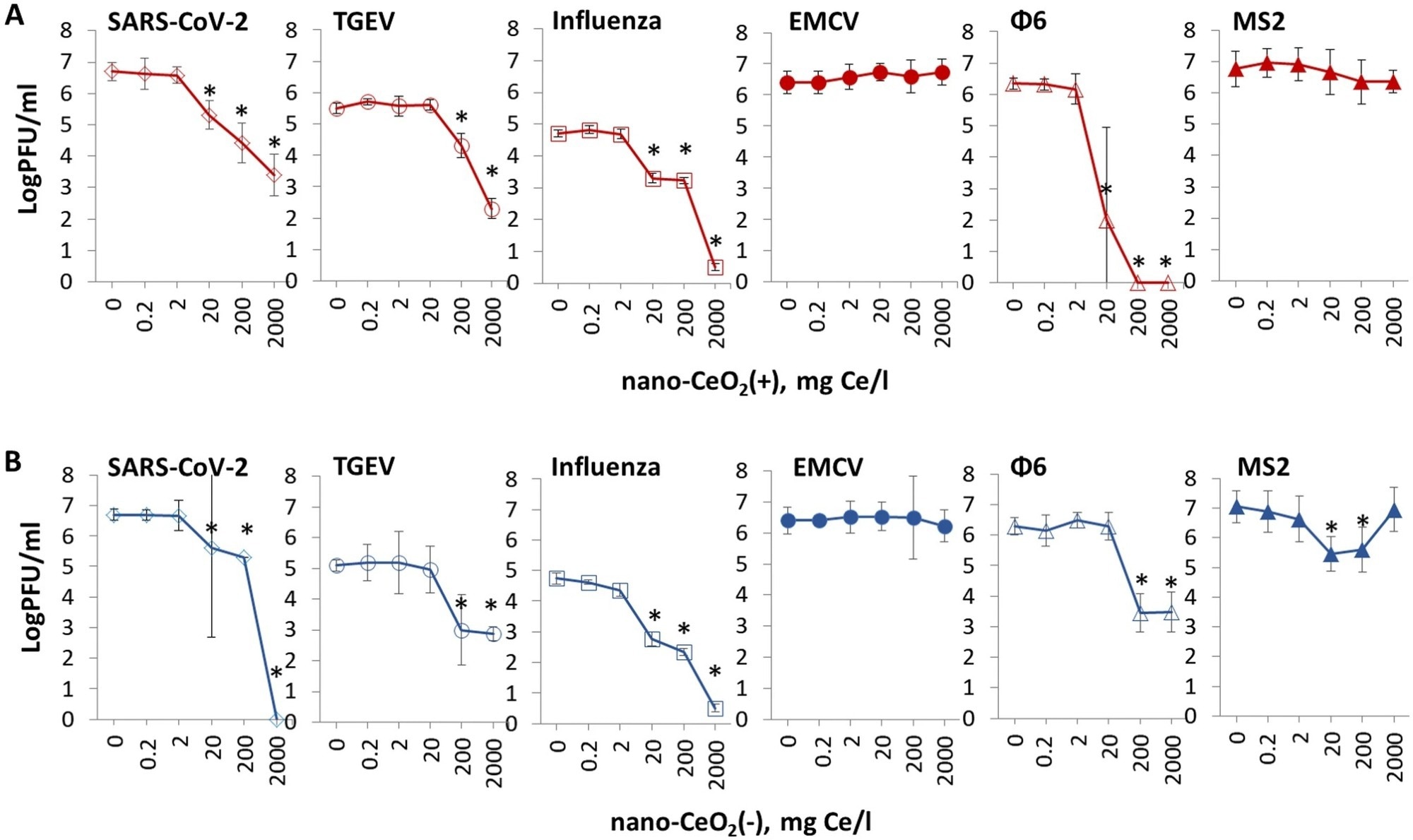
Figure 2. The effect of nano-CeO2(+) (A) and nano-CeO2(−) (B) on viral plaque forming activity (log PFU/ml). Enveloped mammalian viruses and bacteriophages are shown with open symbols; closed symbols represent non-enveloped mammalian viruses and bacteriophages. The differences in initial viral titers are caused by different viral yields in laboratory conditions. *p < 0.05. © Nefedova, A., et al. (2022).
Study Demonstrates Decreased Viral Infectivity Upon Nano-CeO2 Exposure
In this research, the authors investigated how exposure to CeO2 nanoparticles reduced viral infectivity. The findings suggested that CeO2 materials might have potent antiviral properties while being relatively safe for human cells and even shielding them from environmental stress.
The examination of the effects of two kinds of CeO2 nanoparticles on four enveloped viruses revealed a greater than two logs (99%) decline in viral infectivity after introduction to nano-CeO2 at 20 to 200 milligrams per liter. A drop of more than four logs (99.99%) after exposure to 200-2000 milligrams per liter was also observed. However, the two non-enveloped viruses were less sensitive to nano-CeO2.
The MS2 bacteriophage demonstrated a small non-monotonous reduction in infectivity upon exposure to one of the evaluated CeO2 nanoparticles. At the same time, the EMCV picornavirus displayed no reduction in infectivity till the highest tested concentration.
Thus, the results demonstrated that CeO2 nanoparticles might interact with other viral envelope components apart from proteins, such as phospholipids, which were lacking in non-enveloped viruses. Also, the two CeO2 nanoparticles displayed almost identical antiviral activity despite having distinct surface characteristics regarding their charges. Such an observation enabled the authors to hypothesize that the antiviral effects reported were caused by the nanoparticles’ inherent factors rather than the surface functional groups or the surface charges.
However, bacteria displayed substantially less susceptibility to CeO2 nanoparticles than viruses. E. coli’s highest viable count reduction was only 97%, and the antibacterial action was non-monotonous. Nano-CeO2 did not affect the Gram-positive S. aureus at any tested concentration. The absence of cytotoxic effects of nano-CeO2 was also significant.
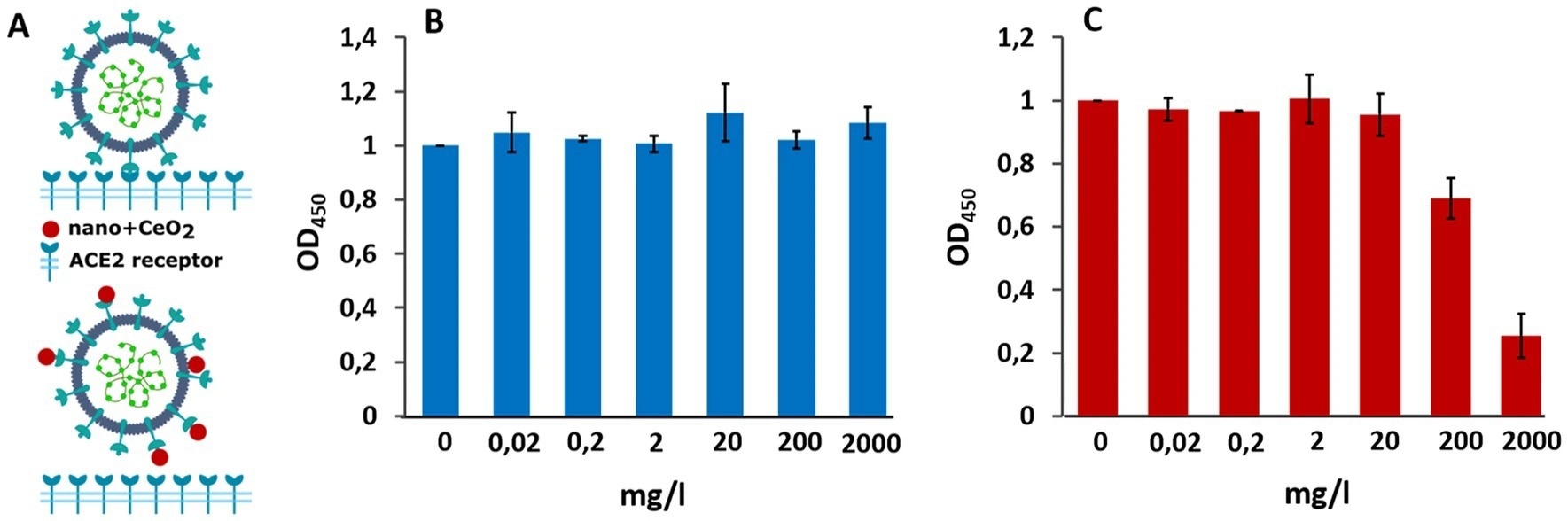
Figure 3. Schematics of the experiment (A), where the upper part shows SARS-CoV-2 binding to ACE2 receptor without nanoparticles and lower part demonstrates the theoretical inhibition of SARS-CoV2 binding to ACE2 receptor by of CeO2 nanoparticles. (B) Shows the effect of nano-CeO2(+) and (C) the effect of nano-CeO2(−) particles on binding of SARS-CoV-2 onto ACE2 receptor in an ELISA assay, measured as optical density (OD450). © Nefedova, A., et al. (2022).
Reference
Nefedova, A., et al. (2022). Antiviral efficacy of cerium oxide nanoparticles. Scientific Reports. https://www.nature.com/articles/s41598-022-23465-6
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.