Transition metal sulfides have great potential for sodium storage due to their high theoretical capacity and abundance. However, low conductivity and volume expansion limit their high-rate performance and cyclic stability.
The schematic illustration of synthetic process of NiS2/FeS. Credit: Energy Material Advances (2023).
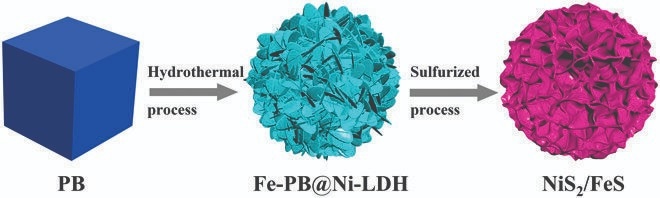
A recent study published in the journal Energy Material Advances focuses on this issue by creating a NiS2/FeS heterostructure as an anode for sodium-ion batteries. This was accomplished by developing layered double hydroxide nanosheets of nickel (Ni) atop Fe-based Prussian Blue nanocrystals.
The resulting nanoparticles have a flower-like structure and may offer improved performance in sodium storage.
Sodium-ion Batteries (SIBs): Importance and Limitations
Renewable energy sources have become increasingly important as society aims to reduce its reliance on finite fossil fuels and combat climate change. Energy storage is a critical component of renewable energy systems, as it enables a reliable and consistent supply of electricity.
Battery technology plays a crucial role in energy storage, and there has been significant research and development in this field in recent years. Sodium-ion batteries (SIBs) are a promising candidate for replacing lithium-ion batteries (LIBs) due to the abundance of sodium on earth, making it a cheaper and more sustainable alternative.
However, SIBs have some drawbacks, including a larger atomic size of sodium compared to lithium, resulting in reduced charge diffusion and volume changes during storage. These challenges can lead to poor cyclic stability and rate performance of SIBs.
Therefore, there is a need to develop unique electrode materials that can improve the electrochemical properties of SIBs and overcome these challenges. By designing these materials, SIBs can become a more viable alternative to LIBs, opening up a new pathway for large-scale energy storage with greater sustainability and lower costs.
Current Anode Materials for Sodium-ion Batteries
The anode materials are critical components of sodium-ion batteries (SIBs) that store energy during the charging process. A range of materials has been studied for their potential use as anode materials in SIBs.
These include transition metal oxides, carbonaceous materials, sulfides, selenides, and metallic alloys. Among these materials, transition metal sulfides have attracted significant attention due to their high theoretical capacity and non-toxicity.
Despite the potential advantages of transition metal sulfides, they still suffer from drawbacks such as large volume expansion and low electronic conductivity. Researchers have employed effective methods such as carbon coating and ion doping to address these issues.
In addition to these strategies, it has been shown that creating heterostructures comprising one n-type and one p-type transistor with distinct band gaps is beneficial in improving the electrochemical characteristics of active materials.
This method creates a high internal electric potential at the border between the two elements, providing a driving force for electron and ion movement at the interface.
Highlights of the Current Research
Using a multistep technique, the researchers created a one-of-a-kind nickel sulfide/iron sulfide (NiS2/FeS) heterostructure in this work. The Fe-PB@Ni-LDH core-shell structure was formed by creating Fe-based Prussian Blue (Fe-PB) nanostructures, which were subsequently coated with nickel-based layered double hydroxide (Ni-LDH) nanosheets.
The nanocomposite was subsequently sulfurized for two hours at 400 °C to produce NiS2/FeS nanostructures with a flower-like shape. The sodium storage capabilities of the NiS2/FeS nanoparticles were also investigated by the researchers.
The increased battery efficiency of the NiS2/FeS heterostructure was demonstrated using the density functional theory (DFT) calculation.
The researchers also investigated the potential for industrial applications of as-synthesized NiS2/FeS. "We investigated the full-cell performance by assembling devices using Na3V2(PO4)3 (NVP) as the cathode and NiS2/FeS as the anode," said paper author Jun Song Chen, with the School of Materials and Energy, the University of Electronic Science and Technology of China. "As-assembled NVP||NiS2/FeS full cell exhibited good electrochemical performance, with good commercial potential."
Key Developments of the Research
The researchers successfully synthesized NiS2/FeS heterostructured nanoflower using Ni-based LDH nanosheets on Fe-PB nanocrystals followed by gas-phase sulfurization.
The as-obtained composite showed high capacity, high-rate performance, and long cycle life for 1,000 cycles, with a low loss per cycle. A full cell consisting of NVP and the as-prepared NiS2/FeS exhibited a reversible capacity and energy density, which was due to the enhanced electrochemical properties of NiS2/FeS offered by the heterostructured interface.
The results of this study show that the design of composite functional materials with heterostructures can be an effective approach for improving material properties for different applications.
The future perspective of this research is to further optimize the design of heterostructured composites and investigate their potential in various fields, such as energy storage, catalysis, and biomedical applications.
The researchers aim to explore the fundamental mechanisms behind the improved properties of heterostructured materials and use this knowledge to design more efficient and durable composite materials for practical applications.
Reference
Yan, D. et al. (2023). NiS2/FeS Heterostructured Nanoflowers for High-Performance Sodium Storage. Energy Material Advances. Available at: https://doi.org/10.34133/energymatadv.0012
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.