The emergence of multi-drug resistance bacteria and healthcare-associated infections are frequent issues in the treatment of suppurating wounds. A group of researchers has created a bactericidal nanomaterial that can be used against either Gram-positive or Gram-negative bacteria. This allows the material to target specific bacteria and successfully fight bacterial diseases.
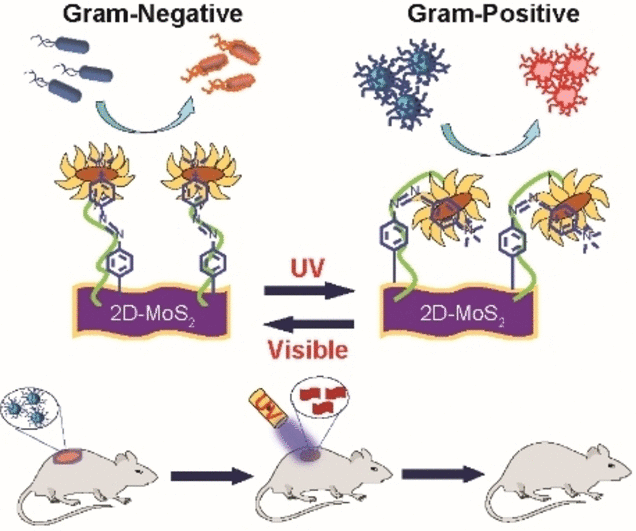
Image Credit: Angewandte Chemie
Its efficacy against MRSA can be extended to other selected bacterial infections, as the team reports in their research published in Angewandte Chemie.
Infections resistant to antibiotics are becoming a serious public health problem, especially in hospital settings. Although many of the bacterial species in concern are common in the wild, in immunocompromised people, they can produce far more severe diseases that are often incurable.
Antibiotics are not the only way to fight healthcare-associated diseases; bactericidal materials provide an alternative. By using a UV-visible light-responsive nanomaterial that can be adjusted to target either Gram-positive or Gram-negative bacteria, Mrinmoy De and colleagues from the Indian Institute of Science in Bengaluru, India, have successfully produced their material.
The content and structure of the outer membranes of the two species of bacteria varies greatly. Methicillin-resistant Staphylococcus aureus (MRSA) and other gram-positive bacteria have bacterial membranes mostly made of peptidoglycans.
The inner and outer membranes of Gram-negative bacteria, on the other hand, are mostly made of phospholipids with a thin coating of peptidoglycan. One such bacterium is Pseudomonas aeruginosa, which is also related to healthcare and has problems with resistance to various antibiotics.
It is important to achieve strain-selective bactericidal activity.
Mrinmoy De, Associate Professor, Department of Organic Chemistry, Chemical Science Division, Indian Institute of Science
The scientists created a functionalized nanomaterial consisting of molybdenum disulfide (MoS2) with azobenzene moieties to which positively charged quaternary amino groups were linked to create a bactericidal agent that could interact with both chemical surfaces preferentially.
The azobenzene moieties induce a light-driven transition in the nanostructure from an elongated trans into a curved cis form to establish selective surface contacts, even though MoS2 is a bactericide and the quaternary amino groups permit membrane depolarization.
The researchers discovered that, albeit in very different ways, the cis and trans variants of the nanomaterial both killed bacteria through the use of a variety of chemical probes and optical measurements. The trans form depolarized and deeply penetrated the bacterial membrane of the Gram-negative P. aeruginosa.
This made it possible for the MoS2 nanomaterial to destroy the bacteria by producing intracellular reactive oxygen species. On the other hand, the cis version was more successfully absorbed by the Gram-positive MRSA strain. In this instance, certain interactions caused the cell wall to become damaged and burst.
The researchers were able to regulate selectivity for either type of bacterium by merely “flipping” the UV switch from the trans ground state to the cis state. They used mouse models to illustrate the effectiveness of their nanomaterial in effectively curing MRSA-infected lesions. When using the cis reagent, the wounds healed fully after ten days, although regular antibiotic treatment with vancomycin was taking longer.
Journal Reference:
Sahoo, J., et al. (2023) Photo-Controlled Gating of Selective Bacterial Membrane Interaction and Enhanced Antibacterial Activity for Wound Healing. Angewandte Chemie International Edition. https://doi.org/10.1002/anie.202314804.