If left to their own devices, bacteria on the teeth or injured skin can envelop themselves in a slimy scaffolding, forming what is known as a biofilm. These bacteria cause havoc on tissues and are hard to remove because the slime protects them from antibiotic medication. A fresh approach might provide an easy means of dislodging the grime and eliminating the bacteria.
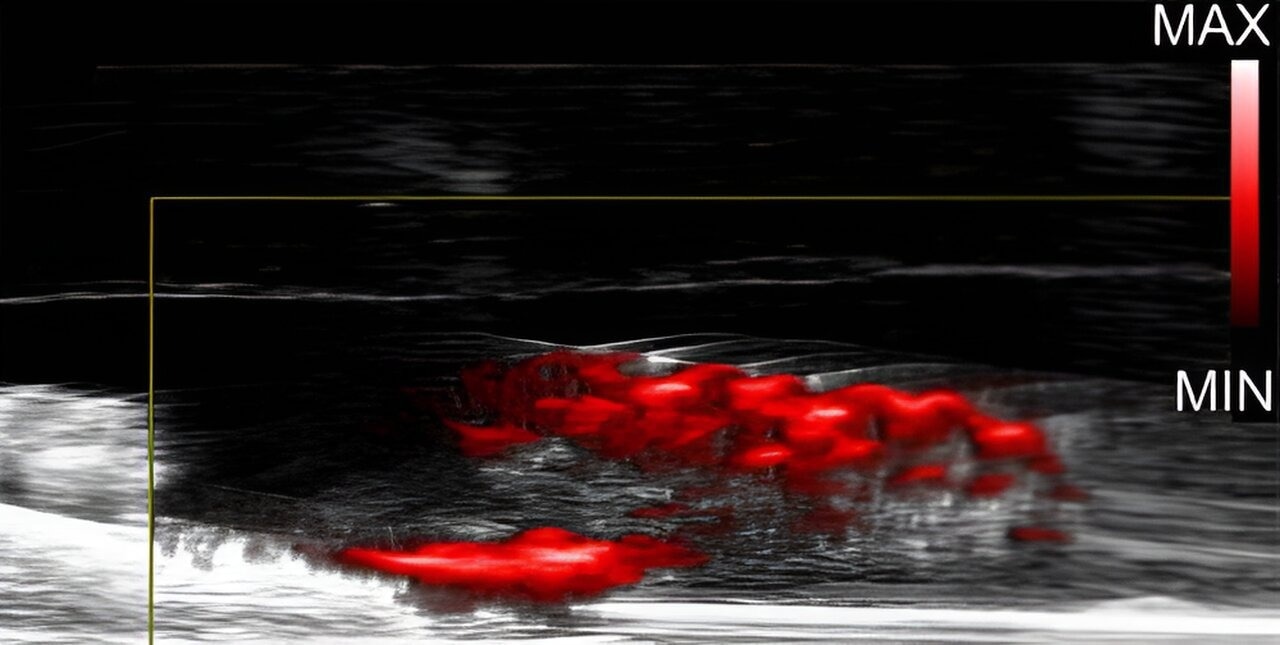 Researchers were able to visualize biofilms in teeth treated with dextran-coated gold nanoparticles using photoacoustic imaging. Image Credit: Journal of Clinical Investigation
Researchers were able to visualize biofilms in teeth treated with dextran-coated gold nanoparticles using photoacoustic imaging. Image Credit: Journal of Clinical Investigation
Scientists at Stanford University and the University of Pennsylvania have created sugar-coated gold nanoparticles that they have used to image and destroy biofilms. Scientists showed how the nanoparticles could be used for diagnostic and therapeutic purposes on the teeth and injured skin of rats and mice. The study was published in the Journal of Clinical Investigation,
They were able to eradicate biofilms in as little as 60 seconds and outperform conventional antimicrobials in this regard.
With this platform, you can bust biofilms without surgically debriding infections, which can be necessary when using antibiotics. Plus, this method could treat patients if they are allergic to antibiotics or are infected by strains that are resistant to medication, and the fact that this method is antibiotic-free is a huge strength.
Luisa Russell, Ph.D., Program Director, Division of Discovery Science & Technology, NIBIB.
Bacteria like Streptococcus mutans can form oral biofilms, or plaques, which can lead to serious tooth decay. The common cause of wound infections, Staphylococcus bacteria, can cause significant delays in the healing process.
Regardless of the situation, the tightly knit web of proteins and carbohydrates found in biofilms can stop antibiotics from getting to all of the bacteria in the afflicted area.
However, that is not all that biofilms pose as a problem. Not only are they challenging to get rid of, but they are also difficult to identify in the first place.
This new research has found that gold is the answer that can solve both issues at once.
Gold is an excellent choice for photothermal therapy, a tactic that uses the heat from nearby pathogens to kill them because it is nontoxic and easily transforms energy from light sources into heat. Gold particles can be seen using a method known as photoacoustic imaging because, in addition to producing heat, the nanoparticles react to light by emitting detectable ultrasonic waves.
The authors of the new study optimized the response of gold spheres to light for both therapeutic and imaging applications by encasing them within larger golden cage-shaped nanoparticles. They covered the particles in dextran, a common carbohydrate used to form biofilms, to make them attractive to bacteria.
By placing the gold nanoparticles on top of S. mutans-infected teeth from ex vivo rat jaws, the researchers evaluated their approach.
The researchers were able to determine exactly where biofilms had absorbed the dextran-coated particles on the teeth thanks to the nanoparticles' clear and loud signals during a photoacoustic imaging test on the teeth.
They then used a laser to irradiate the teeth to assess the particles' therapeutic effect. They applied the topical antiseptic chlorhexidine to other infected tooth samples as a point of comparison.
The researchers saw a clear difference between the two treatments' results: photothermal therapy killed biofilms almost entirely, whereas chlorhexidine had no discernible effect on the viability of the bacteria.
The treatment method is especially fast for the oral infection. We applied the laser for one minute, but really in about 30 seconds we are killing basically all of the bacteria.
Maryam Hajfathalian, Ph.D., Professor and Study First Author, Department of Biomedical Engineering, New Jersey Institute of Technology
Maryam Hajfathalian conducted this study as a postdoctoral researcher at the University of Pennsylvania and Stanford University.
Tests on Staphylococcus aureus-infected mice with open skin wounds were also successful because the heat produced by the nanoparticles significantly outperformed the effects of gentamicin, another antimicrobial agent.
Here, the temperature increased by 20 °C and was limited to the biofilm. It did not appear to harm the surrounding tissue, according to the researchers' measurements.
The authors state that they hope to demonstrate whether the method can stop cavities or hasten healing through additional testing.
I think it is important to see how inexpensive, straightforward, and fast this process is. Since we are limited in using antibiotics, we need novel treatments like this as a replacement.
Maryam Hajfathalian, Ph.D., Professor and Study First Author, Department of Biomedical Engineering, New Jersey Institute of Technology
Journal Reference:
Hajfathalian, M., et al (2023) Theranostic gold-in-gold cage nanoparticles enable photothermal ablation and photoacoustic imaging in biofilm-associated infection models. Journal of Clinical Investigation. doi.org/10.1172/JCI168485