Reviewed by Lexie CornerMay 9 2024
A collaborative research team from Kawasaki Institute of Industrial Promotion (KIIP) and Leiden University developed a new method for reliably and quickly diversifying the reactive end-groups on poly(2-oxazoline)s, a class of biocompatible polymers. The study is published in the Angewandte Chemie International Edition journal.
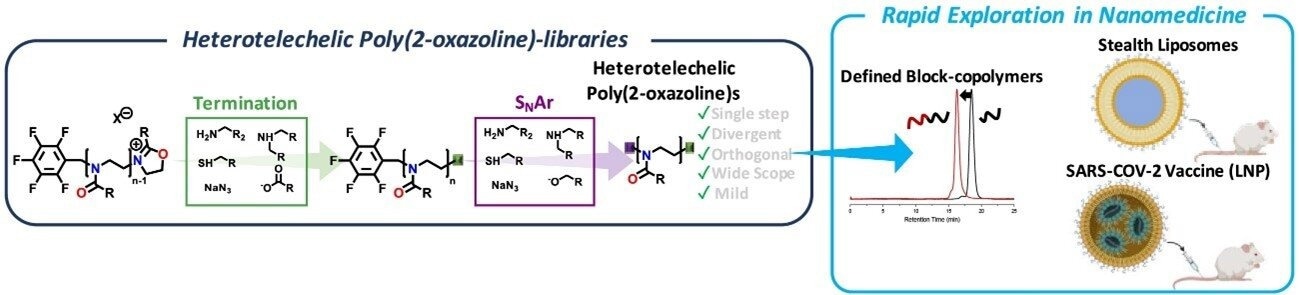 Outline of the synthetic approach, as well as the exploration of the polymers in prototype structures, employ. Image Credit: Innovation Center of NanoMedicine
Outline of the synthetic approach, as well as the exploration of the polymers in prototype structures, employ. Image Credit: Innovation Center of NanoMedicine
The method allows for quick exploration of the potential of poly (2-oxazoline)s in nanomedicine applications. By substituting poly (ethylene glycol) (PEG), it can be used to adjust the pharmacokinetics of nanomedicine and offer potential treatments to patients with contraindications against PEG.
In the synthesis of nanomedicine, reactive end-groups on non-immunogenic biocompatible polymers, like PEG, are commonly employed. The biocompatible polymer increases blood circulation time and stability, allowing passive accumulation at neovascular sites while preventing premature blood clearance and off-target toxicity.
Often referred to as “PEGylation,” the introduction of a biocompatible “stealth” polymer is a standard procedure in the field. This approach has produced lipid nanoparticle (LNP)- mediated SARS-CoV-2 vaccines (e.g., Comirnaty, BioNTech/Pfizer), FDA-approved PEG-protein conjugates (e.g., Peginterferon alfa-2a), and PEGylated liposomes (e.g., Doxil).
These technologies mainly rely on synthesizing PEG with reactive end-groups, which requires several steps.
As a possible substitute for PEG, poly(2-oxazoline)s (POx), a class of biocompatible polymers with great structural versatility, are being studied because their structure can be adjusted to control pharmacokinetics and -dynamics without causing patients to develop immune reactions specific to PEG.
Despite these encouraging characteristics, the creation of libraries containing POx with two distinct reactive chain ends was frequently laborious from a synthetic perspective, necessitating iterative component synthesis or chemistry with a narrow focus, which partially prevented widespread POxylation.
The authors used commercially available pentafluorobenzyl bromide or tosylate initiators for the polymerization to enable simple end-group diversification of POx. This allowed for selective termination using O-, N-, and S-nucleophiles and a subsequent para-fluoro nucleophilic aromatic substitution of the pentafluorobenzyl group using O-, N-, and S-nucleophiles.
The wide range of substrates allows for the easy introduction of different functional moieties, which is appealing for engineering nanoscale drug/gene delivery platforms. The authors showed that their method allowed for the quick synthesis of POx-lipid conjugates, which were then investigated in liposomes and LNP-mediated mRNA delivery. The performance of these systems was not significantly impacted by the addition of a plurifluorophenyl linker.
Inspired by these findings, these lipids were investigated in the administration of SARS-CoV-2 spike mRNA and contrasted with their PEGylated counterparts. Both showed strong immunological responses, suggesting that POx may be a good substitute for PEG.
A Facile End-Group Diversification Approach
Water-soluble, biocompatible, and functional polymers are fundamental building blocks of therapeutic formulations and compounds that allow for enhanced gene/drug delivery and safety profiles by lowering side effects or lowering the frequency of required administration. The biocompatible polymer class known as poly(2-oxazoline)s allows for significant structural versatility and fine-tuning of pharmacokinetic and -dynamic properties.
However, the lack of readily available end-group diversification strategies is impeding the exploration of these properties in nanomedicine.
Using the orthogonal reactivity of an electrophilic 2-oxazolinium species and an electrophilic pentafluorobenzyl group (the reactive chain-end in POx polymerization), a straightforward, one-step end-group diversification method is presented.
The method allows for synthetic diversification with superior end-group fidelity and control over the molecular weight distribution, using a wide range of commercially accessible substrates, including O-, N-, and S-nucleophiles.
The method facilitated swift investigation into the creation of nanomedicine platforms, as demonstrated by the creation of lipid nanoparticles, liposomes, and block-copolymers based on POx for mRNA delivery.
The transfection ability of the POx-based lipid nanoparticles was similar to that of their PEGylated counterparts. Compared to a PEG-control, the prophylactic effect of the SARS-CoV-2 vaccination was unaffected, underscoring the potential of this polymer platform and the chemistry offered.
The study is significant because it provides a simple end-group diversification strategy that speeds up the development of POx-based nanomedicine platforms by ensuring that the synthesized products meet the stringent quality standards of PEG products that are sold commercially.
As a result, POxylated nanomedicine can be investigated quickly, enabling precise pharmaceutical property tuning by choosing the proper polymer structure.
The Innovation Center for NanoMedicine (iCONM; Center Director: Prof. Kazunori Kataoka), a research institute of the Kawasaki Institute of Industrial Promotion (KIIP), and Leiden University (Assistant Prof. Joachim F. R. Van Guyse) collaborated on the study.
Journal Reference:
Van Guyse, R. F. J., et al. (2024) Facile generation of heterotelechelic poly(2-oxazoline)s towards accelerated exploration of poly(2-oxazoline)-based nanomedicine. Angewandte Chemie International Edition.doi.org/10.1002/anie.202404972.