Historically, pharmaceutical drugs are carefully designed at the atomic level to ensure effectiveness and safety, as seen with ibuprofen. Now, researchers at Northwestern University and Mass General Brigham suggest applying this same precision to nanomedicines, which currently lack uniform structure. By tailoring nanomedicines more precisely, scientists aim to create more consistent and potent treatments for major diseases. The study was published in the journal Nature Reviews Bioengineering.
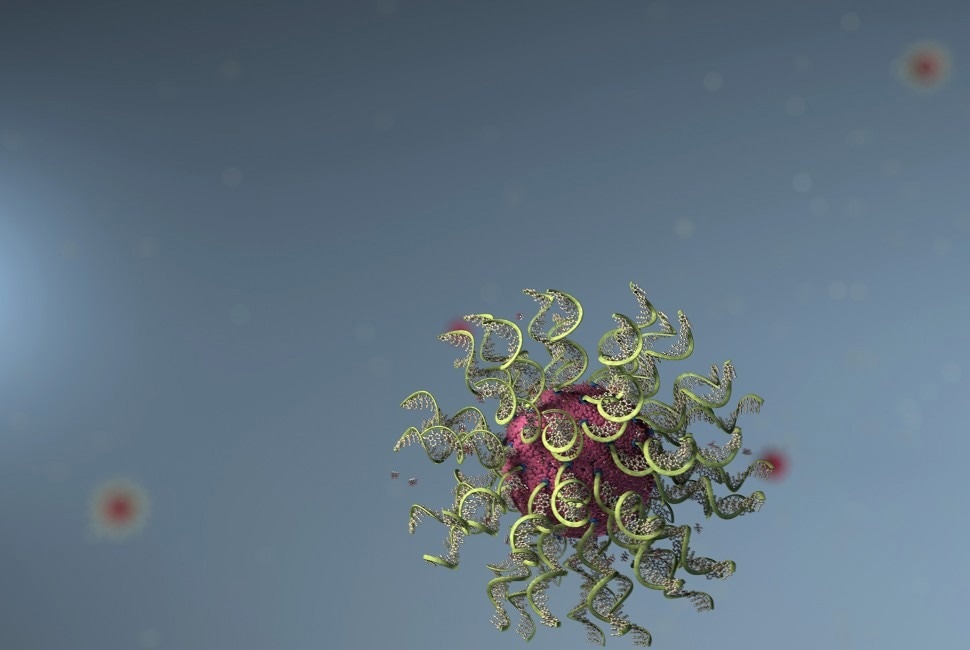 Invented by Chad A. Mirkin, spherical nucleic acids (SNAs) are one example of structured nanomedicine. SNAs are a globular form of DNA that can easily enter cells and bind to targets. Image Credit: Chad A. Mirkin/IIN/Northwestern University
Invented by Chad A. Mirkin, spherical nucleic acids (SNAs) are one example of structured nanomedicine. SNAs are a globular form of DNA that can easily enter cells and bind to targets. Image Credit: Chad A. Mirkin/IIN/Northwestern University
No two particles are alike in today's nanomedicines, such as mRNA vaccines. To guarantee that all of the nanomedicines in the same batch are identical, and the strongest versions researchers are coming up with new ways to precisely customize their structures.
With this degree of control, scientists can precisely regulate how nanomedicines interact with the human body. Strong vaccines or even treatments for cancer, infectious diseases, neurodegenerative diseases, and autoimmune disorders are being developed as a result of these novel designs.
Historically, most drugs have been small molecules. In the small molecule era, it was critical to control the placement of every atom and every bond within a particular structure. If one element were out of place, it might render the whole drug ineffective. Now, we need to bring that tight control to nanomedicine. Structural nanomedicine represents a massive shift in how we can approach therapeutic development.
Chad A. Mirkin, Study Co-Author, Northwestern University
Chad A. Mirkin said, “By focusing on the intricate details in our therapeutics and how different medicinal components are displayed within a larger structure, we can design interventions that are more effective, more targeted and, ultimately, more beneficial for patients.”
As the George B. Rathmann Professor of Chemistry, Chemical and Biological Engineering, Biomedical Engineering, Materials Science and Engineering, and Medicine at Northwestern, where he holds appointments at the McCormick School of Engineering, Feinberg School of Medicine, and Weinberg College of Arts and Sciences, Mirkin is a pioneer in the field of nanomedicine. He is also the founding director of the International Institute for Nanotechnology (IIN).
Mirkin co-authored the perspective with Natalie Artzi, Head of Structural Nanomedicine at the Gene and Cell Therapy Institute at Mass General Brigham, Associate Professor of Medicine at Harvard Medical School, and Core Faculty Member at the Wyss Institute for Biologically Inspired Engineering at Harvard University and Milan Mrksich, Henry Wade Rogers Professor of Biomedical Engineering at McCormick, Professor of Chemistry at Weinberg, and Professor of Cell and Developmental Biology at Feinberg.
Problems with “The Blender Approach” to Vaccine Design
Scientists have primarily depended on combining essential elements in traditional vaccine design methods.
For instance, typical cancer immunotherapies include an adjuvant, which is a molecule that stimulates the immune system, combined with one or more molecules from tumor cells, known as antigens. Doctors create a cocktail by combining the adjuvant and antigen, which they then inject into the patient.
This method, which Mirkin calls the “blender approach,” involves unstructured components. On the other hand, antigens and adjuvants can be arranged using structural nanomedicines.
When structured at the nanoscale, the same medicinal ingredients show improved efficacy and fewer adverse effects compared to their unstructured counterparts. However, in contrast to small-molecule medications, these nanomedicines are still imprecise at the molecular level.
No two drugs in a batch are the same. Nanoscale vaccines have different numbers of lipids, different presentations of lipids, different amounts of RNA, and different sizes of particles. There are an infinite number of variables in nanomedicine formulations. That inconsistency leads to uncertainty. There is no way to know if you have the most effective and safest construct among the vast number of possibilities.
Chad A. Mirkin, Study Co-Author, Northwestern University
Moving from Co-Assembly to Molecular Precision
Mirkin, Mrksich, and Artzi support a move toward even more precise structural nanomedicines as a solution to this issue. This method uses chemically well-defined core structures that can be precisely engineered with several therapeutic components in a regulated spatial arrangement to create nanomedicines.
By managing design at the atomic level, researchers can achieve previously unheard-of capabilities, such as combining several functions into a single drug, optimizing target engagement, and triggering drug release in particular cells.
The paper's authors give three examples of innovative structural nanomedicines: mega molecules, chemo flares, and spherical nucleic acids (SNAs). Developed by Mirkin, SNAs are spherical structures of DNA that readily penetrate cells and attach to specific targets. They are more efficient than linear DNA of the same sequence and have shown great promise in drug delivery, gene editing, gene regulation, and vaccine development. In some clinical settings, they have even been shown to cure fatal types of skin cancer.
Mirkin said, “We have proven that the overall structural presentation of an SNA-based vaccine or therapeutic, not simply the active chemical components, dramatically affects its potency. This finding could lead to treatments for many different types of cancer. In certain cases, we have used this to cure patients who could not be treated with any other known therapy.”
Chemoflares are intelligent nanostructures developed by Artzi and Mirkin that react to disease-related cues in cancer cells to release chemotherapeutic medications. Mrksich created megamolecules, which are expertly put-together protein structures that resemble antibodies. All of these structural nanomedicines can be engineered by researchers to contain a variety of therapeutic agents or diagnostic instruments.
By harnessing disease-specific tissue and cellular cues, next-generation nanomedicines can achieve highly localized and timely drug release, transforming how and where therapies act within the body. This level of precision is especially critical for combination treatments, where coordinated delivery of multiple agents can dramatically enhance therapeutic efficacy while reducing systemic toxicity and minimizing off-target effects.
Natalie Artzi, Head of Structural Nanomedicine, Gene and Cell Therapy Institute, Mass General Brigham
Artzi said, “Such smart, responsive systems represent a crucial step forward in overcoming the limitations of conventional drug delivery.”
Harnessing AI in Design
According to the authors, future research must address present issues with delivery, scalability, reproducibility, and the integration of multiple therapeutic agents. The authors also stress how crucial new technologies like artificial intelligence (AI) and machine learning are becoming for improving design and delivery parameters.
Mirkin said, “When looking at structure, there are sometimes tens of thousands of possibilities for how to arrange components on nanomedicines. With AI, we can narrow down giant sets of unexplored structures to a handful to synthesize and test in the lab. By controlling structure, we can create the most potent medicines with the lowest chance of side effects.”
Mirkin said, “We can restructure medicinal components like nucleic acids to create entities that have properties that go so far beyond what we have ever seen with standard DNA and RNA. This is just the beginning, and we are excited to see what is next. We are poised to usher in an entire new era of structural medicine, with Northwestern taking the lead.”
The study was funded by the National Cancer Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, Edgar H. Bachrach through the Bachrach Family Foundation, the CZ Biohub, the Defense Threat Reduction Agency, and The Lefkofsky Family Foundation.
Journal Reference:
Mirkin, C. A., et al. (2025) The emerging era of structural nanomedicine. Nature Reviews Bioengineering. doi.org/10.1038/s44222-025-00306-5.