Jul 28 2008
In a conventional sewage works, nanoparticles should really be bound in the sludge and should not represent a major problem in the aqueous effluent. This is not true, however, as shown by a new study of the ceramic model material cerium dioxide. An astonishing amount was able to leave an experimental sewage works and thus could possibly enter bodies of water.
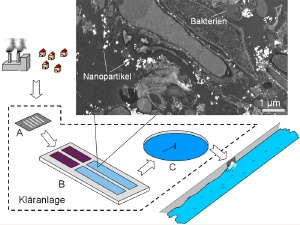 A sewage works consists of a preliminary physical clarification stage (A), biological clarification by bacteria (B) and a chemical clarification stage (C). In this study the behaviour of nanoparticles in the biological clarification stage was investigated in a scalable pilot plant (6 litres). Top right: electron microscope image of cerium oxide nanoparticles (white dots) in sewage sludge. Relatively small agglomerates bind to bacteria.
A sewage works consists of a preliminary physical clarification stage (A), biological clarification by bacteria (B) and a chemical clarification stage (C). In this study the behaviour of nanoparticles in the biological clarification stage was investigated in a scalable pilot plant (6 litres). Top right: electron microscope image of cerium oxide nanoparticles (white dots) in sewage sludge. Relatively small agglomerates bind to bacteria.
The industry needs large amounts of cerium dioxide (CeO2) to grind computer components and mobile phone camera lenses or the lasers in CD players. Thousands of tons of this substance are used throughout the world. But what happens when this or other nano-substances get into the environment, especially sewage, and thereby enter sewage works? Is the problem solved because nanoparticles largely agglomerate, i.e. clump together?
Particles survive unbound
Not at all, according to a new study by the research group led by Wendelin Stark, Assistant Professor at the Institute for Chemical and Bioengineering, which has just appeared in the leading scientific journal “Environmental Science and Technology”. The majority of the cerium dioxide particles actually do bond to the surface of bacteria in the sewage sludge and can thus be removed from the water. However, far more particles than was assumed are able to pass through the biological separation stage. Stark says, “Intuitively, we presumed that practically 100 percent of the cerium oxide particles would remain trapped in the sewage sludge. After all, the particles should form clumps and sink to the bottom.” Therefore at the start of the project they had thought that all the particles could simply be precipitated out.
However, through numerous experiments in a test sewage plant, the scientists discovered that up to six percent by weight of the particles entered the sewage works effluent. Since the experiments were carried out at relatively high nanoparticle concentrations (100 ppm), the researchers suspect that agglomeration, i.e. clumping together, is likely to be impeded even more at lower concentrations. Therefore oxide nanoparticles that get into the effluent must not be regarded as unproblematic, because the route taken by the particles after the sewage works has scarcely been researched up to now.
Bacteria help nanoparticles
The researchers at ETH Zurich, the University of Applied Sciences Wädenswil and the BMG Engineering AG Company then began a series of experiments to find out how the oxide nanoparticles pass through the sewage plant almost unimpeded. Here again, the researchers met with a surprise. Stark stresses that, “The particles disperse astonishingly well and do not agglomerate entirely as was assumed.” The bacteria living in the activated sludge involuntarily share responsibility for this dispersion. They excrete soap-like substances to avoid forming clumps with one another. However, these anti-clumping agents and other components of the aqueous effluent also stabilise the nanoparticles. Thus a proportion of the material is not sufficiently agglomerated and can leave the sewage works practically unchanged.
For Wendelin Stark, this research work is a stroke of luck from which all the participants have profited greatly. The work arose from an initiative by co-author Robert Bereiter, a member of the staff of the University of Applied Sciences Wädenswil, and René Gälli of BMG Engineering. The researchers were able to carry out the tests in the company’s own experimental sewage plant under real conditions and in accordance with OECD standards. The sewage sludge for the experiments originated from the activated sludge tanks of the Werdhölzli plant, Zurich’s biggest sewage works.
Disposed of with the sewage sludge
Elisabeth Müller and Roger Wepf from the Electron Microscopy Centre (EMEZ) of ETH Zurich prepared electron microscope images of sewage sludge with nanoparticles, showing impressively that the nanoparticles accumulate at the surface of the bacteria. At the same time, a large proportion of the nanoparticles remains in the sewage sludge. In Switzerland this sludge is incinerated, but in other countries it is still used as fertiliser.
Cerium dioxide is a popular model compound for studies of this kind, and is also used industrially in rapidly increasing amounts. Titanium oxide, a white pigment and an ingredient in many suncreams, behaves in a similar way to cerium dioxide and is already used in huge quantities. Under the experimental conditions both substances are stabilised in a way similar to that in a sewage works. Other soluble nanoparticles, e.g. iron oxide, are stabilised to a distinctly lesser extent. This is why they are also easier to remove from the environment. Wendelin Stark says, “However, we are still at the beginning of nano-safety research. Further investigations must follow.”
Literature reference
Limbach LK, Bereiter R, Müller E, Krebs R, Gälli R, Stark WJ. Removal of Oxide Nanoparticles in a Model Wastewater Treatment Plant: Influence of Agglomeration and Surfactants on Clearing Efficiency. Environ. Sci. Technol., 2008 June 25, published online, doi: 10.1021/es800091f