Oct 29 2008
Carbon nanotubes (CNTs)-which resemble tiny rolls of chicken wire-are used in electronics, optics and other products because of their unusual strength and electrical conductivity. CNT's are also being used for drug delivery. But an engineer and a chemist affiliated with the Johns Hopkins Institute for NanoBioTechnology have teamed up to study the ways that nanotubes could transport harmful toxins in aquatic environments.
 Oxidized carbon nanotubes with sorbates. Credit: Ball Lab / JHU
Oxidized carbon nanotubes with sorbates. Credit: Ball Lab / JHU
William Ball, professor of environmental engineering in the Whiting School of Engineering, and Howard Fairbrother, professor of chemistry in the Krieger School of Arts and Sciences, received two separate grants from the National Science Foundation and the Environmental Protection Agency to study the effects of surface oxides on the behavior of carbon nanotubes and their influence on the mobility of contaminants in aquatic environments.
"When people or animals drink—or otherwise process—water that has been contaminated by CNTs, they may receive the toxins as well as the CNTs," says Ball. "Retention and toxicity of the CNT-bound chemicals is still unclear, but the retained chemicals and/or the CNTs themselves may cause harm and can also propagate further up the food chain."
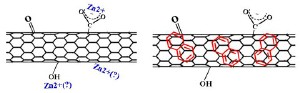
The team will study how the surface chemistry of CNTs-namely the oxygen-containing functional groups (surface oxides) on the nanotubes—influence the material's ability to grab onto, transport, and release organic and inorganic pollutants and metals in lakes, streams and oceans, making the carbon nanotubes behave like a "Trojan Horse."
Part of the study will rely on models based on what is already known about the interaction of oxidized CNT surfaces and toxins. In a study published in Environmental Science and Technology in March 2008, Ball and Fairbrother investigated how surface oxides influenced the adsorption of Naphthalene on multi-walled carbon nanotubes (See reference below). Naphthalene is a common ingredient in mothballs, and exposure to high concentrations of the chemical can damage or destroy red blood cells.
In the experimental phase, the team will oxidize fresh CNTs with nitric acid to mimic the modifications used to purify and functionalize this carbon-based material. Next, the CNTs will be added to columns of silica or sand, and solutions containing organic compounds or metal ions will be flowed through. The liquid that flows out the other end of the column will be collected and analyzed. Testing will occur under different pHs and concentrations of dissolved organic matter, to represent aquatic environments.
These results, Ball says, will be further analyzed in light of appropriate theoretical models, as well as to experimental data about the sorption properties of the carbon nanotubes for various chemicals and the surface-surface interactions among and between CNTs and other materials.
To learn more about the participating Labs visit the profiles in the INBT Faculty Finder.
Reference
Influence of Surface Oxides on the Adsorption of Naphthalene onto Multiwalled Carbon Nanotubes. Cho, Hyun-Hee, Smith, Billy A., Wnuk, Joshua D., Fairbrother, D. Howard, and Ball, William P. Environ. Sci. Technol., 42, 8, 2899 - 2905, 2008, 10.1021/es702363e
Story by Mary Spiro