|
Until now, mass-production of organic nanotubes has been very difficult technically, because a large amount of water solvent has been needed for the synthesis of self-assembled organic nanotubes from amphiphilic molecules.
We have developed a convenient mass-synthesis method for nanotubes which uses less than one thousandth of the solvent used in conventional methods, and the time required for the drying process is only a few hours.
Since the nanotubes we have developed enable the encapsulation of functional substances (proteins, metal nanoparticles, etc.) larger than 10 nm, which have been impossible to encapsulate until now, applications to the slow release of medicines and health foods are expected.
Synopsis
The High Axial-Ratio Nanostructure Fabrication Team of the Nanoarchitectonics Research Center (Toshimi Shimizu, Director) of the National Institute of Advanced Industrial Science and Technology (AIST; Hiroyuki Yoshikawa, President) have newly designed and synthesized amphiphilic molecules with hydrophilic and hydrophobic moieties, and have developed a technique for the synthesis of various organic nanotubes of 40-200 nm in inner diameter, 70-500 nm in outer diameter, and several µm in length by self-assembling them in organic solvents. This method needs less than one thousandth of the solvent used by conventional methods, enabling mass-production of organic nanotubes (Figure 1). Unlike carbon nanotubes, organic nanotubes have excellent dispersibility in water, and can incorporate guest substances of over 10 nm in size, such as proteins and nucleic acids. The organic nanotubes can encapsulate even functional substances that are so large that cyclodextrins, produced on a commercial basis as encapsulation substances at present, cannot do. Thus the organic nanotubes are promising for application to various fields such as medical, health, and nanobio technologies.
This research work was displayed in Orgatechno 2006 held at Pacifico Yokohama from July 25-27.
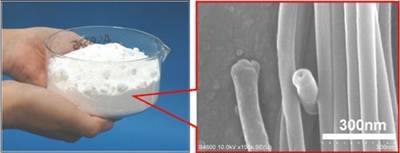
Figure 1. (Left) White solid powders (weight = 100 g) consisting of organic nanotubes (mean outer diameter = 80 nm, and mean inner diameter = 60 nm), and (right) a scanning electron micrograph of the nanotubes we have developed.
Background and History of Research Work
Studies on carbon nanotubes, which consist of only carbon atoms, have been extensively advanced from the view points of application research, practical use, and mass production. On the other hand, there have been organic nanotubes whose outer diameters are similar to multiple-layered carbon nanotubes of ten to several tens of nm in outer diameter. Similar to soap molecules, the organic nanotubes are hollow cylindrical nanostructures formed by spontaneous assembling of amphiphilic molecules with a water-soluble (hydrophilic) and oil-soluble (hydrophobic) moieties. This assembling is called "self-assembly." It has been found that only a limited kind of amphiphilic molecules, such as phospholipids, glycolipids, and peptidelipids, can self-assemble into nanotube structures.
The sizes of organic nanotubes are usually 10-200 nm in inner diameter, 40-1000 nm in outer diameter, and several to several hundreds µm in length, although depending on the amphiphilic molecules used. The molecules form cylindrical bilayer structures, in which the hydrophilic groups of the molecules are oriented to both surfaces of each bilayer to contact with water (Figure 2). Over several millions molecules are orderly arranged by purely intermolecular interactions without chemical bonding to form stable nanostructure structures.
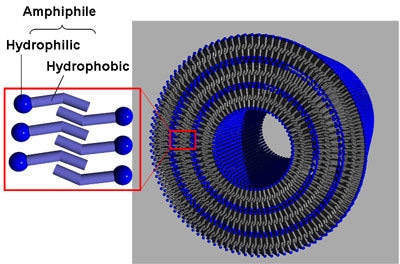
Figure 2. An illustration of typical molecular packing of an organic nanotube.
The head of each tadpole-like amphiphilic molecule indicates the hydrophilic group and the tail indicates the hydrophobic group.
There have been self-assembly methods in water for the synthesis of organic nanotubes, but the methods have the disadvantage that a large amount of water, corresponding to 1000-10000 times the weight of the organic nanotubes, is needed. Furthermore, the methods require many steps and a long time to finally transform into nanotube structures (Figure 3). Thus, until now, it has been considered that the mass-production of over 1 gram of nanotubes is difficult at the laboratory level.
AIST has advanced the study of the design, synthesis, and self-assembly of amphiphilic molecules for nanotube formation over the last decade, and in this work we have succeeded in the mass-production of organic nanotubes. This work was carried out as part of the Core Research for Evolutional Science and Technology (CREST) project, the joint research of the Japan Science and Technology Agency (JST) and AIST (in the FY of 2000-2005), and in the part of the Solution Oriented Research for Science and Technology (SORST) project, the contract research of JST (in the FY of 2005-2007).
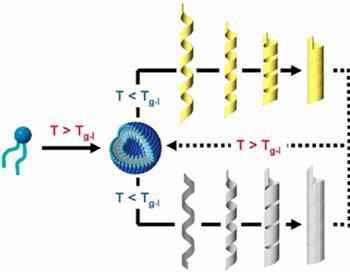
Figure 3. A morphological transformation mechanism of amphiphilic molecular assemblies, first forming spherical assemblies, then going through helical assemblies, and finally resulting in nanotube-like structures in water.
Details of Research Work
In this work, using the hydrophilic and hydrophobic parts of inexpensive and safe materials such as saccharides and peptides which can be used as foods, we have designed and synthesized N-glycoside-type glycolipids and peptidelipids for the formation of nanotubes. Moreover, we have succeeded in the synthesis of hollow fiber-like organic nanotubes by self-assembly of the lipids in safe organic solvents, such as ethanol which is also used for food, but not using a water solvent.

Figure 4. A possible schematic diagram showing a self-assembly mechanism of our amphiphilic molecules in an organic solvent (drawn by the Research Institute of Nanotechnology).
By convenient processing, such as room-temperature preservation and evaporation of solvents, and using organic solvents which are good solvents of nanotube materials, we have succeeded in mass-producing over 1 kg of solid-powdery organic nanotubes with the amounts of solvents lower than 1,000-10,000th of that needed for conventional methods.
Figure 4 illustrates that the synthesized amphiphilic molecules can form nanotube assemblies in only one step, without undergoing multiple steps like nanotubes in water, resulting in the production of a large amount of nanotubes in a very short time. We have confirmed that the white solid powders consist of organic nanotubes of 40-200 nm in inner diameter, 70-500 nm in outer diameter, and several µm in length using transmission and scanning electron microscopes (Figure 5).
In this work, we have produced over 1 kg of nanotubes using organic solvents of approximately 10 L (conventional methods need 20,000 L of water). Furthermore, in preparation of nanotubes enabling encapsulation of functional substances, conventional methods need a vacuum-drying process over several days, but our organic solvent method makes the drying process easy to accomplish in several hours.
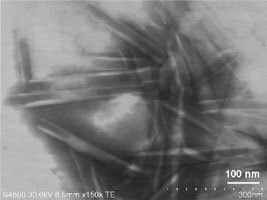
Figure 5. A scanning electron microscopic image (transmission mode) of white organic nanotubes in the solid powdery state.
The characteristics, sizes, and functions of our organic nanotubes are different from those of carbon nanotubes, and hereafter their applications, research and development, and research for practical use will be accelerated as a work originating from Japan. We have thus named our nanotubes the "Organic Nanotube AIST," and we have recently applied for this to be registered as our trademark.
Cyclic molecules, called "cyclodextrin," which are constituted of 6-8 glucose molecules connected circularly, have been widely used in a variety of fields, such as food, medicine, and house hold goods. Encapsulating various organic low-molecular-weight compounds in their hydrophobic pockets, the molecules have functions in making unstable substances stable, in the slow release of medicines and aroma chemicals, and in making water-insoluble substances soluble.
On the other hand, organic nanotubes formed by self-assembly of glycolipids can be well dispersed in water. Furthermore, the nanotubes can encapsulate 10-50-nm scale substances, e.g. proteins, nucleic acids, viruses, and metal nanoparticles, which cyclodextrin molecules cannot, to disperse them in water. Actually, using organic nanotubes of 30-60 nm in inner diameter, we have succeeded in the encapsulation of metal nanoparticles of 1-20 nm in diameter and spherical proteins of 12 nm in diameter (ferritin) as shown in Figure 6.
Recently products utilizing encapsulation functions of cyclodextrin have been researched and developed, and many of them have already been produced on a commercial basis. However, our nanotubes, enabling mass-production and encapsulation of large molecules, are promising for industrial applications as new materials with encapsulation functions.
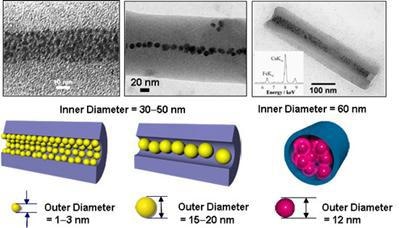
Figure 6. (Left and center) Transmission electron micrographs of nanotubes with inner diameters of 30-50 nm, encapsulating gold nanoparticles with different sizes, respectively. (Right) a transmission electron micrograph of a nanotube with an inner diameter of 60 nm, encapsulating ferritin with an outer diameter of 12 nm.
Future Plans For Research Work
Hereafter, we plan to advance the development of organic nanotubes from the view point of new nanotube containers or new organic nanotube carriers with the capability of adsorption, encapsulation, and slow release, considering applications to fields of (1) agriculture (the removal of prions, slow-release manure, etc.), (2) foods (discharge of fat, functional fibers, etc.), (3) health (prevention of alopecia, allergen filters, etc), (4) medical care (drug delivery systems for target regions, hemocatharsis, capturing of viruses, administration of insulin, atomization, etc), (5) environment (removal of metallic particulates, etc), and (6) health food additive materials for women and the elderly.
|