Solid lipid nanoparticles (SLNs) serve as an alternative carrier system to traditional colloidal carriers like polymeric microparticles, nanoparticles, liposomes and emulsions. SLNs act as a new colloidal drug carrier for intravenous applications. They are in the submicron size range of 50 to 1000 nm and are formed of biocompatible lipids, which are solid at room temperature, dispersed in water or an aqueous surfactant solution.
The biocompatibility of the lipid matrix is a major factor that allows SLNs to be used as drug carriers. SLNs combine the benefits of liposomes, fat emulsions and polymeric nanoparticles. SLNs can be produced using homogenization techniques, spray congealing methods and through microemulsions.
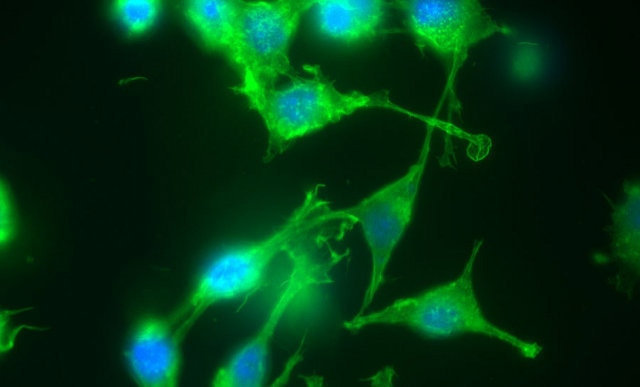
Solid lipid nanoparticles are absorbed by mounse dendritic cells. The nanoparticles are labelled with a green fluorescent protein marker, and the nuclei of the cells appear blue. Image credit: Mumper Lab, Carolina Institute for Nanomedicine.
Properties of Solid Lipid Nanoparticles
Some of the major properties of SLNs are detailed below:
- High drug loading capacity
- Large surface area
- Small in size
- More stable than biological liposomes
- Readily biodegradable
- Less toxic than ceramic or polymer nanoparticles
Nanoparticle Drug Delivery
Derlivery systems for various drugs have already been developed using SLNs. Loading capacity is the major factor that determines the efficiency of a drug carrier system. However, the loading capacity can be influenced by the polymorphic state of the material, the chemical and physical structure of the solid lipid matrix, miscibility of lipid and drug melt and drug solubility in melted lipid. The incorporated drug concentration can be estimated using centrifugation filtration and by determining entrapment efficiency - extensive testing using these and other methods is required to establish the efficacy of any SLN-based delivery system.
Although there is little information available about drug releasing mechanisms, studies have determined a few basic principles associated with drug release. More effective drug release can be achieved with smaller sized particles, which provide a larger surface area. Some of the other factors that contribute to rapid release of drug are low viscosity in the matrix, short diffusion distance for the drug and high diffusion co-efficient. Meanwhile, slow drug release is accomplished by the homogenous dispersion of drug in the lipid matrix. Drug release from solid lipid nanoparticles and other carrier systems can be assessed by various in vitro and ex vivo methods.
Recent Developments
Generally, adjuvants are used in vaccines to improve the immune response. One of the new developments in the adjuvant sector is SLNs that slowly degrade to ensure prolonged exposure to the immune system. The extremely small size of SLNs is beneficial for drug targeting. SLNs have the capability to penetrate the blood-brain barrier for treating central nervous system disorders. These nanoparticles are evaluated for lymphatic uptake upon intraduodenal administration to rats. They have also been proved to be successful upon encapsulating anti tubercular drugs in tuberculosis chemotherapy.
Delivery of drugs to the specific organs or sites is the biggest challenge in pharmaceutical sciences. However, with the use of colloidal delivery systems like SLNs, improved and precise drug delivery can be achieved. Recent studies have suggested that SLN when incorporated with essential oil from Artemesia arboreseens L can be used as a convenient carrier for safe pesticides. In addition, cationic SLNs are being formulated for gene transfer with the help of cationic lipids that control the in vitro transfection performance.
Conclusion
A number of nanoscale drug delivery systems have been engineered over the last two decades, and solid lipid nanoparticles are among the most important of them due to the different administration routes they allow and unique characteristics. SLNs have the potential to reach the right target site in the body, at the right concentration without any side effects. Although SLNs are complex systems with their own pros and cons, further research has to be carried out to obtain a clear model of the structure and dynamics of drug release at the molecular level.
References
- "Lipid nanoparticles: state of the art, new preparation methods and challenges in drug delivery" - Luigi Battaglia & Marina Gallarate, Informa Healthcare, 2012. DOI: 10.1517/17425247.2012.673278
- "Solid lipid nanoparticles: Production, characterization and applications" - Wolfgang Mehnert & Karsten Mäder, Advanced Drug Delivery Reviews, 2001. DOI: 10.1016/S0169-409X(01)00105-3
- "Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art" - Rainer H. Mueller, Karsten Maeder, Sven Gohla, European Journal of Pharmaceutics and Biopharmaceutics, 2000. DOI: 10.1016/S0939-6411(00)00087-4