Mar 16 2009
Single oxygen atoms dancing on a metal oxide slab, glowing brighter here and dimmer there, have helped chemists better understand how water splits into oxygen and hydrogen. In the process, the scientists have visualized a chemical reaction that had previously only been talked about. The new work improves our understanding of the chemistry needed to generate hydrogen fuel from water or to clean contaminated water.
 With the help of a single oxygen atom, the titanium oxide surface helps a water molecule split into two oxygen-hydrogen pairs called hydroxyls. Credit: Lyubinetsky/PNNL
With the help of a single oxygen atom, the titanium oxide surface helps a water molecule split into two oxygen-hydrogen pairs called hydroxyls. Credit: Lyubinetsky/PNNL
The scientists made the discovery while trying to determine the basics of how titanium dioxide -- a compound sometimes found in sunscreen -- breaks down water. The chemical reactions between water and oxygen are central to such varied processes as hydrogen production, breaking down pollutants, and in solar energy.
"Oxygen and water are involved in many, many reactions," said physicist Igor Lyubinetsky at the Department of Energy's Pacific Northwest National Laboratory, who reported the team's results in March 6 issue of the Physical Review Letters. "This mobility might interfere with some reactions and help others."
Bustling Bright Spots
While exploring titanium dioxide as a way to split water into its hydrogen and oxygen pieces, researchers can use a technique called scanning tunneling microscopy to watch the chemical reaction. The surface of a slab of titanium dioxide is like a corn field: rows of oxygen atoms rise from a patch of titanium atoms. The alternating oxygen and titanium rows look like stripes.
Scientists can also see some atoms and molecules that come to rest on the surface as bright spots. One such visible atom is a single oxygen atom that comes to rest on a titanium atom, called an "adatom". Chemists can only see water molecules if they drop the temperature dramatically -- at ambient temperature, water moves too fast for the method to pick them up.
In this work, PNNL scientists studied water's reactions with titanium dioxide at ambient temperature at EMSL, the DOE's Environmental Molecular Sciences Laboratory on the PNNL campus. Starting with a surface plated with a few oxygen adatoms, they added water --and the adatoms started to dance.
"Suddenly, almost every adatom started to move back and forth along the titanium row," said Lyubinetsky. "From theory and previous work, we expected to see this along the row."
Remarkably, the adatoms didn't just slide up and down the stripes. They also bounced out of them and landed in others, like pogoing dancers in a mosh pit.
"We saw quite unexpected things. We thought it was very strange -- we saw adatoms jump over the rows," Lyubinetsky said. "We just couldn't explain it."
Calculating how much energy it would take for the adatoms to move by themselves, much less hop over an oxygen row, the chemists suspected the adatoms were getting help -- most likely from the invisible water molecules.
The Unseen Enabler
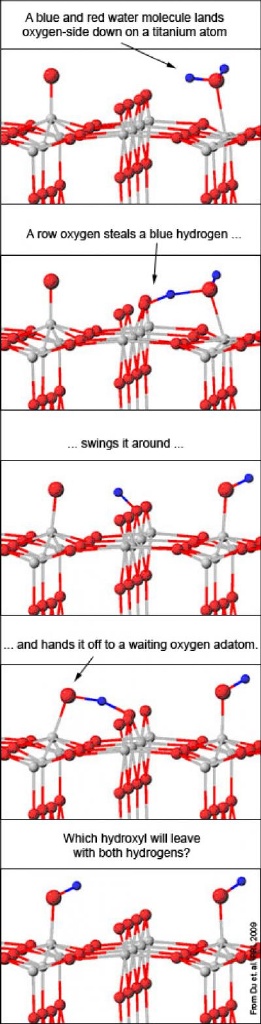
To make sense of the dancing adatoms, the team calculated how much energy it would take to move adatoms with the help of water molecules. If a water molecule sits down next to an adatom, one of the water's hydrogen atoms can jump to the adatom, forming two oxygen-hydrogen pairs.
These pairs are known as hydroxyls and tend to steal atoms from other molecules, including each other. One of the thieving hydroxyls can then nab the other's hydrogen atom, turning back into a water molecule. The water molecule floats off, leaving behind an adatom. Half the time, that adatom is one spot over -- which makes the original appear to have moved.
The chemists determined that water can help the adatom jump a row as well: If a water molecule and an adatom are situated on either side of a raised oxygen row, a row oxygen can serve as the middleman, handing over a hydrogen from the water molecule to the adatom. Again, two hydroxyls form, one ultimately stealing both hydrogens (with the help of the middleman) and zipping away as water. If the incoming water molecule has been stripped, the adatom appears to have hopped over.
The calculated energy required for these different scenarios fit well with the team's experimental data. When a row oxygen serves as a middleman, the process is known as "pseudo-dissociation", a reaction suggested by chemists but until now, never verified experimentally.
"We realized that only if we involved the pseudo-dissociative state of the water can we explain it," said Lyubinetsky. "Otherwise, all the calculations show there's too high a barrier, the adatom just cannot jump by itself."
Lyubinetsky points out that this shows that water itself can work as a catalyst. A catalyst is a molecule that can help a chemical reaction along and remain unchanged by the experience.
"Water is required to move the adatoms around, but like a catalyst it is not consumed in the reaction," he said. "You start with water and you end with water."
In the future, the team plans on determining if water can make the adatoms move other species and more than one space at a time. In addition, they will investigate how light affects the reaction.