Mar 19 2009
In order to save money and energy, many people are purchasing hybrid electric cars or installing solar panels on the roofs of their homes. But both have a problem -- the technology to store the electrical power and energy is inadequate.
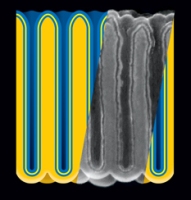 Electrostatic nanocapacitors formed in nanoporous anodic aluminum oxide (darker yellow) film by sequential atomic layer deposition of metal (blue), insulator (yellow), and metal. Insert: cross-section of actual structure, represented as rescaled scanning electron micrograph. (A. James Clark School of Engineering", University of Maryland)
Electrostatic nanocapacitors formed in nanoporous anodic aluminum oxide (darker yellow) film by sequential atomic layer deposition of metal (blue), insulator (yellow), and metal. Insert: cross-section of actual structure, represented as rescaled scanning electron micrograph. (A. James Clark School of Engineering", University of Maryland)
Battery systems that fit in cars don't hold enough energy for driving distances, yet take hours to recharge and don't give much power for acceleration. Renewable sources like solar and wind deliver significant power only part time, but devices to store their energy are expensive and too inefficient to deliver enough power for surge demand.
Researchers at the Maryland NanoCenter at the University of Maryland have developed new systems for storing electrical energy derived from alternative sources that are, in some cases, 10 times more efficient than what is commercially available. The results of their research are available in the latest issue of Nature Nanotechnology.
"Renewable energy sources like solar and wind provide time-varying, somewhat unpredictable energy supply, which must be captured and stored as electrical energy until demanded," said Gary Rubloff, director of the University of Maryland's NanoCenter. "Conventional devices to store and deliver electrical energy -- batteries and capacitors -- cannot achieve the needed combination of high energy density, high power, and fast recharge that are essential for our energy future."
Researchers working with Professor Rubloff and his collaborator, Professor Sang Bok Lee, have developed a method to significantly enhance the performance of electrical energy storage devices.
Using new processes central to nanotechnology, they create millions of identical nanostructures with shapes tailored to transport energy as electrons rapidly to and from very large surface areas where they are stored. Materials behave according to physical laws of nature. The Maryland researchers exploit unusual combinations of these behaviors (called self-assembly, self-limiting reaction, and self-alignment) to construct millions -- and ultimately billions -- of tiny, virtually identical nanostructures to receive, store, and deliver electrical energy.
"These devices exploit unique combinations of materials, processes, and structures to optimize both energy and power density -- combinations that, taken together, have real promise for building a viable next-generation technology, and around it, a vital new sector of the tech economy," Rubloff said.
"The goal for electrical energy storage systems is to simultaneously achieve high power and high energy density to enable the devices to hold large amounts of energy, to deliver that energy at high power, and to recharge rapidly (the complement to high power)," he continued.
Electrical energy storage devices fall into three categories. Batteries, particularly lithium ion, store large amounts of energy but cannot provide high power or fast recharge. Electrochemical capacitors (ECCs), also relying on electrochemical phenomena, offer higher power at the price of relatively lower energy density. In contrast, electrostatic capacitors (ESCs) operate by purely physical means, storing charge on the surfaces of two conductors. This makes them capable of high power and fast recharge, but at the price of lower energy density.
The Maryland research team's new devices are electrostatic nanocapacitors which dramatically increase energy storage density of such devices - by a factor of 10 over that of commercially available devices - without sacrificing the high power they traditionally characteristically offer. This advance brings electrostatic devices to a performance level competitive with electrochemical capacitors and introduces a new player into the field of candidates for next-generation electrical energy storage.
Where will these new nanodevices appear? Lee and Rubloff emphasize that they are developing the technology for mass production as layers of devices that could look like thin panels, similar to solar panels or the flat panel displays we see everywhere, manufactured at low cost. Multiple energy storage panels would be stacked together inside a car battery system or solar panel. In the longer run, they foresee the same nanotechnologies providing new energy capture technology (solar, thermoelectric) that could be fully integrated with storage devices in manufacturing.
This advance follows soon after another accomplishment, the dramatic improvement in performance (energy and power) of electrochemical capacitors (ECC's), thus 'supercapacitors,' by Lee's research group, published recently in the Journal of the American Chemical Society. Efforts are under way to achieve comparable advances in energy density of lithium (Li) ion batteries but with much higher power density.
"The University of Maryland's successes are built upon the convergence and collaboration of experts from a wide range of nanoscale science and technology areas with researchers already in the center of energy research," Rubloff said.
The Research Team
Gary Rubloff is Minta Martin Professor of Engineering in the materials science and engineering department and the Institute for Systems Research at the University of Maryland's A. James Clark School of Engineering. Sang Bok Lee is associate professor in the Department of Chemistry and Biochemistry at the College of Chemical and Life Sciences and WCU (World Class University Program) professor at KAIST (Korea Advanced Institute of Science and Technology) in Korea. Lee and Rubloff are part of a larger team developing nanotechnology solutions for energy capture, generation, and storage at Maryland. Their collaborators on electrical energy storage include Maryland professors Michael Fuhrer (physics), associate director of the Maryland Nanocenter Reza Ghodssi (electrical and computer engineering), John Cumings (materials science engineering), Ray Adomaitis (chemical and biomolecular engineering), Oded Rabin (materials science and engineering), Janice Reutt-Robey (chemistry), Robert Walker (chemistry), Chunsheng Wang (chemical and biomolecular engineering), Yu-Huang Wang (chemistry) and Ellen Williams (physics), director of the Materials Research Science and Engineering Center at the University of Maryland.
This work was partially supported by the Laboratory for Physical Sciences and by the university's Materials Research Science and Engineering Center under a grant from the National Science Foundation.