Apr 24 2009
The actin protein exists in two major forms in the cell: as individual molecules of globular (G)-actin, or linked together as long filaments of fibrous (F)-actin. Actin microfilaments provide the primary scaffolding for contractile muscle fibers, and act as a key component of cellular infrastructure in general. However, many cell types also derive their mobility from directional microfilament growth and disassembly, a process powered in part by the hydrolysis of energy-providing adenosine triphosphate (ATP) molecules.
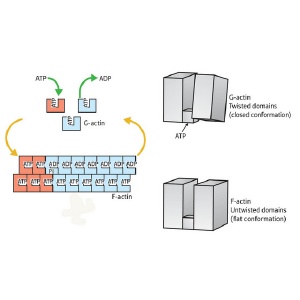 Overview of the actin assembly/disassembly process. G-actin (top right) is bound by ATP (top right) and undergoes a transition into the F-state, enabling it to assemble into fibers (bottom left). This transition involves rotation of the two major domains into a ’flattened’ state (bottom right). This shift also enables ATP hydrolysis (top left), subsequently driving subunit dissociation and a return to G-actin conformation.
Overview of the actin assembly/disassembly process. G-actin (top right) is bound by ATP (top right) and undergoes a transition into the F-state, enabling it to assemble into fibers (bottom left). This transition involves rotation of the two major domains into a ’flattened’ state (bottom right). This shift also enables ATP hydrolysis (top left), subsequently driving subunit dissociation and a return to G-actin conformation.
Although crude structural data on actin have been available for well over a decade, these have proven insufficient to provide a detailed understanding of the mechanism of the G-to-F-actin transition and ATP binding and hydrolysis. As such, a high-resolution structure of F-actin published recently in Nature by RIKEN SPring-8 Center researcher Toshiro Oda and his colleagues represents an important leap forward in understanding the function of this essential protein*.
Their findings revealed a major structural difference between the two states, an overall ‘flattening’ of the actin molecule as it enters the F state, brought about by a large shift in the relative positioning of the protein’s two segments. “A simple rotation of the two major domains of the actin molecule is the essence of the G- to F-actin transition,” explains Oda. “This simple rotation produces the flat conformation.”
In the current model for microfilament assembly, the association of ATP with F-actin has a stabilizing effect, while the hydrolysis of ATP by actin’s enzymatic subdomain is believed to destabilize the F-actin and induce localized disruption of the microfilament. The data from Oda’s team indicate that the flattening of F-actin not only drives fiber polymerization, but also leads to other internal rearrangements favorable to subsequent ATP hydrolysis, although additional analysis will be required to confirm this.
*Oda, T., Iwasa, M., Aihara, T., Maeda, Y. & Narita, A. The nature of the globular- to fibrous-actin transition. Nature 457, 441–445 (2009).