May 4 2009
In future, cartilage, tendon and blood vessel tissue will be produced in the laboratory, with cells being grown on a porous frame, such as non-wovens. A new software program helps to characterize and optimize the non-wovens.
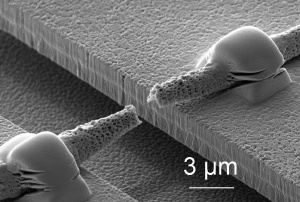 Torn single fiber after a tensile test in the micro-testing machine
© Fraunhofer IWM
Torn single fiber after a tensile test in the micro-testing machine
© Fraunhofer IWM
When someone’s knee hurts with every step it’s a sign that the cartilage has been so badly damaged that the bones rub together when walking. Medical scientists are developing a technique to produce cartilage tissue artificially so that patients with such knee problems can walk free of pain again. The aim is also to make tendons and blood vessels in the laboratory. The research scientists place cells on a porous scaffold material, for example a non-woven made of polymer fibers. The cells can then grow on this frame and form tissue. Whether the cells will grow properly into tissue, however, depends on many factors. For instance, the cells only form cartilage if they are subjected to loads comparable with those in the body. To form cartilage the tissue needs to experience the pressure applied by every step. By contrast, blood vessel tissue needs the pulsation of the blood. The scientists reproduce these loads in the cell culture. When the artificial cartilage is inserted in the patient’s knee the supporting scaffold is gradually resorbed and only the cartilage tissue remains.
While it is quite easy to produce npn-wovens from thin polymer fibers, it is difficult to describe these materials experimentally and theoretically. What forces do the cells experience when the non-woven is pulled or when a liquid passes through the fibre network? How do cells penetrate the non-woven? How do liquids permeate the non-woven? Research scientists at the Fraunhofer Institute for Mechanics of Materials IWM in Freiburg and Halle have developed a simulation model which answers these questions and characterizes the fleeces. “The simulation reproduces the mechanical properties of the fleeces and the transport processes – the software can therefore also calculate how nutrients are transported to the cells and metabolic products are transported away from the cells when a liquid flows by,” explains Dr. Raimund Jaeger, group manager at the IWM. “Understanding these processes can be helpful for cell culture.” To produce the model, the research scientists initially studied the mechanical properties of the individual polymer fibers and for this purpose developed a special apparatus. On a silicon chip measuring one square centimeter, the scientists in Halle etched approximately 50 “microtesting machines”. They then placed and fastened the fibers over the testing machines. Under the microscope the researchers were able to observe how the fibers behave when they are pulled, how far they stretch and when they snap. As fiber-like structures are frequently encountered in nature and technology, suitable experimental techniques and simulation methods have a wide range of applications.