Apr 20 2010
Scientists and engineers have used uniform magnetic fields to drive iron-bearing nanoparticles to metal stents in injured blood vessels, where the particles deliver a drug payload that successfully prevents blockages in those vessels.
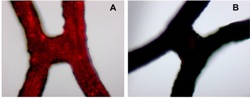 Two hours after local delivery of fluorescently labeled magnetic nanoparticles, the red areas indicate significantly larger amounts of nanoparticles in vascular stents in the presence of a magnetic field (A) compared to no magnetic field (B)
Two hours after local delivery of fluorescently labeled magnetic nanoparticles, the red areas indicate significantly larger amounts of nanoparticles in vascular stents in the presence of a magnetic field (A) compared to no magnetic field (B)
In this animal study, the novel technique achieved better results at a lower dose than conventional non-magnetic stent therapy.
Conducted in cell cultures and rats, the research is the latest in a series of studies at The Children’s Hospital of Philadelphia demonstrating the feasibility of magnetically guided nanoparticles as a new delivery platform for a variety of possible therapeutic cargos: DNA, cells and drugs. The findings may set the stage for a new medical tool, called vascular magnetic intervention.
“This can become a major platform technology for delivering drugs and other agents to specific sites where they can produce benefits in diseased or injured blood vessels,” said study leader Robert J. Levy, M.D., the William J. Rashkind Endowed Chair in Pediatric Cardiology at The Children’s Hospital of Philadelphia.
The research appears in the Proceedings of the National Academy of Sciences, published online this week. Levy’s group from Children’s Hospital collaborated with engineers and scientists from Drexel University, Northeastern University and Duke University.
Levy’s work introduces a new delivery system to an existing medical technology—catheter-deployed stents. Patients with heart disease commonly receive such stents, narrow metal scaffolds that widen a partly clogged blood vessel. These stents are often coated with antiproliferative drugs such as paclitaxel. Paclitaxel inhibits the accumulation of smooth muscle cells within the stent that cause an obstruction.
However, current drug-eluting stents have their limitations. They contain a fixed dose of medication, good for just one release. In a significant number of patients, reobstruction occurs. Levy’s magnetically guided system broadens the possibilities for stents, since magnetic targeting permits using higher doses, redosing if problems recur and using more than one type of agent to treat a blood vessel with a stent.
Levy made use of nanotechnology—the application of extremely small materials. His lab team created nanoparticles, approximately 290 nanometers across, made of a biodegradable polymer and impregnated with magnetite, an iron oxide. (A nanometer is one millionth of a millimeter; these nanoparticles are ten to 100 times smaller than red blood cells.). The magnetite in the particles responds strongly to a magnetic field. Being biodegradable, the particles break down safely in the body after releasing their payload.
Levy’s team first implanted stainless steel stents into the carotid arteries of live rats. After injecting paclitaxel-loaded nanoparticles into the rat’s arteries through a catheter, they produced a uniform magnetic field around each rat for five minutes. The magnetic field, comparable to that produced by existing MRI machines, but one-tenth as strong, magnetized both the stents and the nanoparticles, and drove the particles into the stents and the nearby arterial tissue.
The researchers inserted stents and nanoparticles into a group of control rats, but without using a magnetic field. Five days after receiving the nanoparticle infusion, the magnetically treated animals had four to 10 times as many particles in their stented arteries as the control animals.
Moreover, using magnetic fields to concentrate the treatment had a lasting effect. Fourteen days after using the magnetic field and a single dose of magnetic nanoparticle-encapsulated paclitaxel, the researchers found the rat arteries had significantly lower restenosis than found in arteries of control rats that had no magnetic treatment.
Over the past several years, Levy and colleagues have shown similar proofs of concept in other animal studies, using magnetically guided nanoparticles to deliver gene therapy and therapeutic endothelial cells to arterial stents. The technique is versatile, Levy says, adding that it could also deliver a broad range of effective therapeutic agents.
Stents and magnetic fields might also deliver combination therapies. Nanoparticles could carry different agents simultaneously or at different times. Since the stents remain in place, physicians could retreat patients, delivering therapeutic agents through catheters under magnetic guidance. Because the magnetic effect concentrates its delivery package at the specific site of a stent, physicians could achieve stronger effects with lower overall doses of a given agent. Contributing to the technique’s efficiency, the polymer-based nanoparticles provided sustained drug release over the 14-day course of the study.
Levy envisions a future therapy called vascular magnetic intervention, in which a patient would receive regular treatments from a vascular surgeon or interventional cardiologist who delivers doses of therapeutic nanoparticles under a low-level, uniform magnetic field.
Although currently stents are primarily used for heart patients, Levy cited a great unmet need among the millions of patients with chronic peripheral artery disease. In diabetes patients with poor circulation, for example, drug-eluting stents have had “disappointing results,” Levy says, because leg arteries are larger than coronary arteries, and insufficient drug doses are included in the stent coating. “Our technique offers opportunities for a novel approach in which we can vary doses and repeat the treatments,” he adds.
In children, stents are used to mechanically enlarge anatomic structures for conditions such as peripheral pulmonary artery stenosis, the heart defect coarctation of the aorta, and atrial septal defects created by interventional techniques to provide oxygenated blood. Levy suggests the magnetically guided nanoparticles might deliver drugs that could improve the outcomes in each of these settings, as well as a number of other stent-based interventions used in pediatric cardiology.
For the magnetically-guided nanoparticles that Levy studies, potential clinical applications are still in the future, but possibly not too distant. He expects to partner with clinical researchers in the next few years to bring vascular magnetic intervention closer to clinical reality. “This technique is poised to become a new platform for interventional therapies that could be safer and more effective than the current treatments,” he said.
The National Institutes of Health, the American Heart Association and the William J. Rashkind Endowment of The Children’s Hospital of Philadelphia provided funding support for this study. Co-authors with Levy were Michael Chorny, Ph.D., Ilia Fishbein, M.D., Ivan S. Alferiev, Ph.D., and Marina Bakay, of Children’s Hospital; Benjamin B. Yellen, Ph.D., of Duke University; Srinivas Ganta, and Mansoor Amiji, of the School of Pharmacy of Northeastern University; and Gary Friedman, Ph.D., of Drexel University.
“Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields,” Proceedings of the National Academy of Sciences, published online April 2010. www.pnas.org/cgi/doi/10.1073/pnas.0909506107.
Source: http://www.chop.edu/