Sep 29 2010
An $18 million research program headed by Washington University School of Medicine in St. Louis will research therapies and diagnostic tools for heart and lung diseases that use nanotechnology.
The award, from the National Heart, Lung, and Blood Institute, will fund five years of research at Washington University and four collaborating institutions: Texas A&M University, University of Texas Southwestern Medical Center, and the University of California, Santa Barbara and Berkeley.
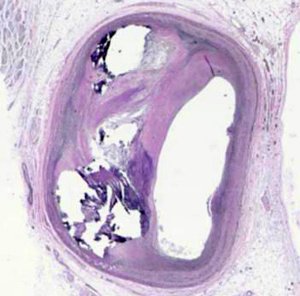 Washington University School of Medicine in St. Louis and other institutions have new funding from the National Heart, Lung and Blood Institute to develop ways to use nanotechnology to treat diseases of the heart and lung. One project will work on nanoparticle-based detection systems for atherosclerotic plaques. Researchers want to use the new system to predict if a plaque like the one above (near the center, to the left of the large white space) might rupture and cause a stroke or heart attack. Pamela Woodard
Washington University School of Medicine in St. Louis and other institutions have new funding from the National Heart, Lung and Blood Institute to develop ways to use nanotechnology to treat diseases of the heart and lung. One project will work on nanoparticle-based detection systems for atherosclerotic plaques. Researchers want to use the new system to predict if a plaque like the one above (near the center, to the left of the large white space) might rupture and cause a stroke or heart attack. Pamela Woodard
Nanoparticles are 1 to 100 billionths of a meter in size. Scientists custom-engineer these tiny particles to deliver imaging agents or therapies, such as drugs, chemotherapies or genetic material to specific targets like tumors, a particular cell type or sites of inflammation.
"Nanoparticles have several advantages over the small molecules typically used in imaging and therapeutics," says Michael Welch, PhD, professor of radiology and developmental biology and co-principal investigator. "Not only can we load them with agents that deliver therapies to specific targets, we can include imaging agents that help us track both the nanoparticles and the therapeutic agent and change the surface of the particles to customize the amount of time they spend in the body."
The new initiative includes four principal research projects.
A synthetic chemistry group, led by Karen Wooley, PhD, of Texas A&M University, will develop targeting molecules that allow nanoparticles to bind with receptors on the surfaces of cells involved in heart and lung diseases. Scientists target nanoparticles to different objectives in the body by customizing the particles' physical properties. This can include both adjustments of the materials nanoparticles are made of and alterations of the crystalline and molecular configurations of the particles' components.
Wooley joins Welch as co-principal investigator of the overall project.
Scientists led by Carolyn Cannon, MD, PhD, of the University of Texas Southwestern Medical Center, will develop nanoparticles as a means of treating patients with cystic fibrosis. This inherited condition subjects patients to repeated, life-shortening lung infections.
Cannon has shown that silver-based therapeutic agents act as antimicrobials and can treat lung infections in mouse models. She has also found that delivering those treatments with nanoparticles increases their effectiveness and reduces the required dosage.
Cannon and her colleagues will use the new funding to complete the preclinical research necessary to begin testing this approach in humans with cystic fibrosis.
A third team of researchers, led by Steven Brody, MD, associate professor of medicine at Washington University, will use nanoparticles to diagnose and treat various forms of acute lung inflammation.
"Trauma and infectious diseases are the most common causes of this inflammation, but there are others, including exposure to hazardous chemicals and additional genetic factors that we don't fully understand yet," Brody says. "We have some simple therapies, such as antibiotics, but to make real progress we need the flexibility and power that nanotechnology can provide, both in diagnosis and therapy."
As proof of principle, Brody and his colleagues will be working to develop ways to use nanoparticles to image and suppress the activity of nitric oxide synthase, an enzyme commonly produced at high levels in inflammatory reactions in the lungs and airway.
The fourth group, led by Pamela Woodard, MD, professor of radiology at Washington University School of Medicine, will work to develop nanoparticles to help physicians detect early atherosclerosis.
Researchers have already created and tested nanoparticles that target a biological indicator that appears very early in the plaque formation process. The indicator is a receptor that blood cell vessels begin producing at higher levels even before plaques start to stabilize.
Preclinical studies have shown such nanoparticle-based agents offer major advantages over the small-molecule agents more typically used to look for plaques. Scientists plan to test the nanoparticle-based imaging agents in Phase I clinical trials in the final years of the award.
"We want to find ways to stratify patients based on risk of heart disease, which could potentially allow us to begin preventive treatments earlier in the disease process," Woodard says.
The award will also fund developmental projects evaluating receptors that can be targeted with nanoparticles to image atherosclerosis and at ways to use optical imaging techniques to track the behavior of nanoparticles.
A nanomaterials production core, led by Craig Hawker, PhD, professor of chemistry, biochemistry and materials at the University of California, Santa Barbara, and director of the UCSB Materials Research Laboratory, will provide facilities for the scaled-up production of promising nanoparticle systems for pre-clinical studies. Facilities at Texas A&M University and Washington University for additional nanoparticle production will provide materials for use in preclinical and clinical trials.
A portion of the funds will support educational programs directed by Carolyn Anderson, PhD, professor of biochemistry and molecular biophysics and of radiology at Washington University School of Medicine. One goal of these programs, targeted to audiences ranging from fourth graders to postgraduate students, will be to stimulate interest in careers in medical nanotechnology development.