Mechanical engineering students from MIT have designed a microfluidic model to explore how cancer cells split from the original tumor and spread into the vascular system of the body.
With the help of this microfluidic device, MIT Professor Roger Kamm and William Polacheck, mechanical engineering student, have found that the metastasizing or spreading of tumor cells throughout the body occurs in the direction in which the body fluids flow through the tissues. Joseph Charest from Charles Stark Draper Laboratory also joined MIT engineers in this study,
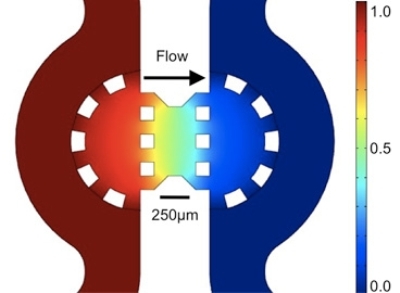 Color map showing the distribution of pressure across the gel region containing the cancer cells
Color map showing the distribution of pressure across the gel region containing the cancer cells
They state that with this new finding, there are chances of restricting the migration of tumor cells. Kamm and Polacheck's microfluidic system enable viewing of interaction between cells and tissue similar to breast tissue, while past approaches only visualized each cell in an artificial extracellular condition.
The study was based on the knowledge that, due to constant growth, fluid pressure is exerted by the cancer cells on the nearby tissues. This high fluid pressure generates a flow in the direction opposite to the tumor. The three dimensional microfluidic system comprises two channels that are separated by a single cell region in a matrix or gel where a flow can be initiated. Polacheck and Kamm experimented on breast-cancer cells. The objective of their study was to simulate the migration process in the body and discovered that the tumor cells showed movement upstream rather than going with the flow, because there were two mechanisms that competed with each other.
The two mechanisms are autologous chemotaxis and the newly discovered one, which occurs in tumor conditions. This mechanism is activated when the flowing fluid pressure along a cell activates receptors known as integrins, thus leading to upstream migration. The microfluidic system could find applications in several biological studies related to liver toxicity, liver disease, inflammation and so on, as it can simulate the cellular interactions within the human body in real time.