Jul 9 2013
As sports injuries go, a torn tendon ranks right up there: searing pain, followed by a protracted period of healing that often lasts for months. If the rupture is severe there's surgery involved, and because of the heavy stresses tendons must endure, a simple reattachment doesn't always hold. Thus the market for tendon replacements.
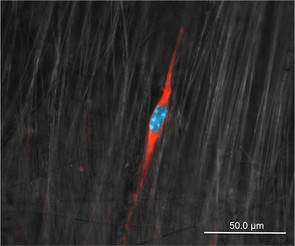 In this scanning electron microscope image, immunostained cells align themselves with polymer nanofibers
In this scanning electron microscope image, immunostained cells align themselves with polymer nanofibers
The required tissue is often excised from a tendon elsewhere in the body, or, after processing, from a cadaver. "The problem with both is limited availability," says Brittany Banik. "That's where our research comes in."
Banik, a graduate student in bioengineering at Penn State, is working with her advisor, assistant professor Justin Brown, on a synthetic replacement for tendon. The challenge is one of basic engineering: achieving the necessary mechanical strength and stability in a new material while keeping its cost reasonable. Banik, who earned her B.S. at Clemson University, received a National Science Foundation graduate research fellowship to pursue this goal at Penn State.
"Engineering tissue requires three components," she says. "First, you need a scaffold. Next, stem cells to regenerate tissue on top of that scaffold. Finally, you typically need bioactive growth factors."
As Brown explains, growth factors are hormones: large proteins that communicate with stem cells to turn on the genes responsible for the desired growth—in this case, tendon cells. "They're very effective," he says. "But they're also very expensive."
Banik and Brown are trying to minimize that expense by manipulating the contours of the tiny polymer fibers that make up the scaffold itself. The right shape, they say, can trigger a stem cell to turn into a tendon cell, just like a growth factor. It may sound implausible, Brown admits, but "interesting things start to happen once you get down to the nanoscale."
As he explains, cells communicate through protein-protein interactions. "Protein A binds to protein B, and eventually the complex ends up in the nucleus and affects some change in gene expression. If you have a substrate that matches the order of magnitude of these protein-protein complexes, then the shape of that substrate can affect the formation of the complexes."
The scale has to be just right, Brown notes. "Over 10-20 microns [roughly a quarter the size of a grain of salt] and it's too big. There's no real mechanism for a stem cell to do anything with something that large. Likewise when you get down below one nanometer—that's smaller than a protein. But when you're in the sweet spot—larger than a couple of proteins and smaller than an organelle—little things make big differences. One of the things we're trying to do is really understand how each element of a nanofiber dictates a response to a cell."
At the same time, on the macro level, Banik and Brown are working on the issue of mechanical strength. Here too, they say, shape is paramount. "It's a matter of graft retention," Brown says. "If you make something flat and sew it in between two ends of tendon, it's easy to tear out those sutures."
Instead, Banik says, "Our scaffold is made up of nanofibers woven into the shape of a tube." In this case, she adds, weaving means laying down criss-crossing strands of fiber in an alternating pattern, rather than actually interlacing them, but "the principle's the same."
Importantly, the design is seamless. Thus, when sewn into place and subjected to the normal stretching a tendon undergoes, the tube contracts instead of pulling apart, its weave compressing to hold things tight. "It works like a Chinese finger trap," Banik says.
Weaving at the nanoscale, however, is no simple matter. In order to achieve the criss-cross pattern, Banik says, she uses a process called electrospinning, in which the polymer is charged at high voltage and extruded under pressure through a syringe; the target is flanked by two magnets to create the fiber alignment. "It's a tricky process," she says. "There are a lot of different components that can affect your end product, and some variables you can't control."
The polymer in question is polycaprolactone, or PCL, a biodegradable polyester commonly used in sutures and root canal filling. As Brown notes, "The idea is that as the PCL scaffold slowly degrades it is replaced by natural tendon tissue grown from the stem cells we've placed on it."
So far, Banik says, "I've been able to spin some tube-shaped scaffolds, and with imaging we've been able to see that the fibers are organized in the proper axial alignment. We've even added stem cells onto these scaffolds to see how they respond. They seem to align themselves with the fibers, so that's another step in the right direction."
The next challenge, she says, is to optimize the diameter of the fibers for maximum effect. "Just by changing that one parameter we have seen huge changes in intracellular communication," Brown notes.
After that, Banik will put the polymer scaffold through mechanical testing, stretching and compressing it like a normal tendon, before conducting final tests in a bioreactor, an environment that replicates conditions within the human body.
That, after all, is the point of all the effort.
"One of the reasons I like bioengineering is that its applications impact people's lives," Banik says. "I hope you won't need a tendon replacement some day. But if you do, I hope we'll have it for you."