Jul 19 2013
Stanford University scientists have created the thinnest, most efficient absorber of visible light on record. The nanosize structure, thousands of times thinner than an ordinary sheet of paper, could lower the cost and improve the efficiency of solar cells, according to the scientists. Their results are published in the current online edition of the journal Nano Letters.
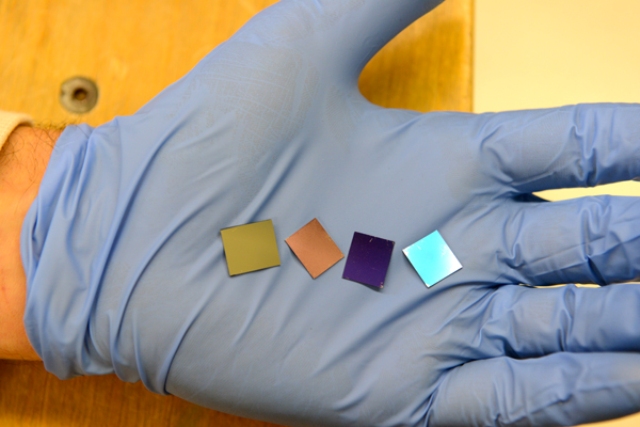 These four wafers contain the thinnest light-absorber ever built. The absorber layer consists of billions of gold nanodots. Each round dot has a volume equivalent to a flat particle of gold 1.6-nanometers thick. Credit: Mark Shwartz
These four wafers contain the thinnest light-absorber ever built. The absorber layer consists of billions of gold nanodots. Each round dot has a volume equivalent to a flat particle of gold 1.6-nanometers thick. Credit: Mark Shwartz
"Achieving complete absorption of visible light with a minimal amount of material is highly desirable for many applications, including solar energy conversion to fuel and electricity," said Stacey Bent, a professor of chemical engineering at Stanford and a member of the research team. "Our results show that it is possible for an extremely thin layer of material to absorb almost 100 percent of incident light of a specific wavelength."
Thinner solar cells require less material and therefore cost less. The challenge for researchers is to reduce the thickness of the cell without compromising its ability to absorb and convert sunlight into clean energy.
For the study, the Stanford team created thin wafers dotted with trillions of round particles of gold. Each gold nanodot was about 14 nanometers tall and 17 nanometers wide.
Visible spectrum
An ideal solar cell would be able to absorb the entire visible light spectrum, from violet light waves 400 nanometers long to red waves 700 nanometers in length, as well as invisible ultraviolet and infrared light. In the experiment, postdoctoral scholar Carl Hagglund and his colleagues were able to tune the gold nanodots to absorb one light from one spot on the spectrum: reddish-orange light waves about 600 nanometers long.
"Much like a guitar string, which has a resonance frequency that changes when you tune it, metal particles have a resonance frequency that can be fine-tuned to absorb a particular wavelength of light," said Hagglund, lead author of the study. "We tuned the optical properties of our system to maximize the light absorption."
The gold nanodot-filled wafers were fabricated at a nearby Hitachi facility using a technique called block-copolymer lithography. Each wafer contained about 520 billion nanodots per square inch. Under the microscope, the hexagonal array of particles was reminiscent of a honeycomb.
Hagglund's team added a thin-film coating on top of the wafers using a process called atomic layer deposition. "It's a very attractive technique, because you can coat the particles uniformly and control the thickness of the film down to the atomic level, " he said. "That allowed us to tune the system simply by changing the thickness of the coating around the dots. People have built arrays like this, but they haven't tuned them to the optimal conditions for light absorption. That's one novel aspect of our work."
Record results
The results were record-setting. "The coated wafers absorbed 99 percent of the reddish-orange light," Hagglund said. "We also achieved 93 percent absorption in the gold nanodots themselves. The volume of each dot is equivalent to a layer of gold just 1.6 nanometers thick, making it the thinnest absorber of visible light on record – about 1,000 times thinner than commercially available thin film solar cell absorbers."
The previous record-holder required an absorber layer three times thicker to reach total light absorption, he added. "So we've substantially pushed the limits of what can be achieved for light harvesting by optimizing these ultrathin, nano-engineered systems," Hagglund said.
The next step for the Stanford team is to demonstrate that the technology can be used in actual solar cells.
"We are now looking at building structures using ultrathin semiconductor materials that can absorb sunlight," said Bent, co-director of the Stanford Center on Nanostructuring for Efficient Energy Conversion (CNEEC). "These prototypes will then be tested to see how efficiently we can achieve solar energy conversion."
In the experiment, the researchers applied three types of coatings – tin sulfide, zinc oxide and aluminum oxide – on different nanodot arrays. "None of these coatings are light-absorbing," Hagglund said. "But it has been shown theoretically that if you apply a semiconductor coating, you can shift the absorption from the metal particles to the semiconductor materials. That would create more long-lived energetic charge carriers that could be channeled into some useful process, like making an electrical current or synthesizing fuel."
Final goal
The ultimate goal, Bent added, is to develop improved solar cells and solar fuel devices by confining the absorption of sunlight to the smallest amount of material possible. "This provides a benefit in minimizing the material necessary to build the device, of course," she said. "But the expectation is that it will also allow for higher efficiencies, because by design, the charge carriers will be produced very close to where they are desired – that is, near where they will be collected to produce an electrical current or to drive a chemical reaction."
The scientists are also considering nanodot arrays made of less expensive metals. "We chose gold because it was more chemically stable for our experiment," Hagglund said. "Although the cost of the gold was virtually negligible, silver is cheaper and better from an optical point of view if you want to make a good solar cell. Our device represents an orders-of-magnitude reduction in thickness. This suggests that we can eventually reduce the thickness of solar cells quite a lot."
Other researchers on the project include Engineering Professor Mark Brongersma and former postdoctoral scholars Isabell Thomann and Han-Bo-Ram Lee from Stanford; and Gabriel Zeltzer and Ricardo Ruiz of Hitachi Global Storage Technologies in San Jose, Calif.
The research was supported by CNEEC, an Energy Frontier Research Center funded by the U.S. Department of Energy. Additional support was provided by the Marcus and Amalia Wallenberg Foundation.
Mark Shwartz writes about energy science and technology for the Precourt Institute for Energy at Stanford University.