Oct 22 2013
NanoSight reports on how Nanoparticle Tracking Analysis, NTA, has been used to demonstrate the product performance of BD Medical - Pharmaceutical Systems' novel cross-linked silicone coating, BD XSi™, used in BD's prefillable syringe BD Neopak XSi™.
Silicone oil is commonly used as a lubricant coating in prefilled syringes (PFS) and is becoming one of the most highly discussed topics in the PFS market, particularly for developers of highly sensitive biotech drugs. In specific biological drug cases, unexpected drug-container interactions have been reported that were potentially attributed to or potentially associated with sub-visible particles generated from the lubricating silicone layer. These could lead to "solution inspection" concerns or even compatibility concern.
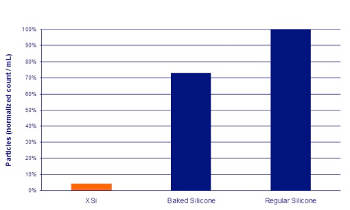 Demonstration of NTA from BD Medical: Sub-micron range particles counts of XSi, baked silicone and conventionally lubricated BD syringes (particle counting, 200nm-12m using a NanoSight NTA system).
Demonstration of NTA from BD Medical: Sub-micron range particles counts of XSi, baked silicone and conventionally lubricated BD syringes (particle counting, 200nm-12m using a NanoSight NTA system).
Sebastien Jouffray is Core Team leader in the R&D Product Development Group at BD Medical – Pharmaceutical Systems. He leads a team working on an innovative immobilised silicone coating, (XSi™). BD XSi™ significantly reduces sub-visible particles (SbVPs) while retaining lubrication performance. BD XSi™ does not introduce any new materials, enabling rapid implementation in PFS for both legacy as well as pipeline drugs.
Detection of SbVPs, defined as particles that cannot be reliably detected by visual inspection with unaided human eye, is continuously being improved by emerging analytical techniques. In particular, the extension of the SbVP size detection limit below 10 µm, potentially into the sub-micron range, and the ability to differentiate the nature of those particles will greatly enhance the potential to monitor and report this critical attribute.
Such analytical techniques can be used to understand better the contribution of silicone lubricant in prefilled syringes, to the overall pool of detected SbVPs. This knowledge will be critical to monitor, control and, if a specific need arises, reduce this contribution.
This need led Jouffray to add new techniques to his arsenal of characterization tools. He used Nanoparticle Tracking Analysis from NanoSight to clearly show that their new coating, BD XSi™, radically reduced the presence of particles compared with traditional baked silicone and conventional sprayed-silicone prefilled syringes.
This data, reported in a focus on injectable drug delivery by ONdrugDELIVERY1, clearly demonstrated that BD XSi™ provides a true reduction in particles in both micron and sub-micron ranges and not just a shift in counts from one range to another.
Noting the benefits of using NanoSight's NTA instrumentation in complement to other characterization techniques, Jouffray said "NanoSight allows for the counting of nano-sized particles in small sample volumes in parallel with other techniques. This gives us cost effective routine characterization technique verifying the quality of BD Neopak XSi™ and for pharmaceutical companies a new technology to count SbVPs in nano range scale".
To find out about the company and to learn more about particle characterization using NanoSight's unique nanoparticle tracking analysis solutions, visit www.nanosight.com and register to receive the next issue of NanoTrail, the company's electronic newsletter.
1. Improving compatibility with biologics with a novel cross-linked silicone coating - S Jouffray, No. 33, July 2012, www.ondrugdelivery.com, Frederick Furness Publishing.
About NanoSight
NanoSight delivers the world's most versatile and proven multi-parameter nanoparticle analysis in a single instrument.
NanoSight's "Nanoparticle Tracking Analysis" (NTA) detects and visualizes populations of nanoparticles in liquids down to 10 nm, dependent on material, and measures the size of each particle from direct observations of diffusion. Additionally, NanoSight measures concentration and a fluorescence mode differentiates suitably-labelled particles within complex background suspensions. Zeta potential measurements are similarly particle-specific. It is this particle-by-particle methodology that takes NTA beyond traditional light scattering and other ensemble techniques in providing high-resolution particle size distributions and validates data with information-rich video files of the particles moving under Brownian motion.
This simultaneous multiparameter characterization matches the demands of complex biological systems, hence its wide application in development of drug delivery systems, of viral vaccines, and in nanotoxicology. This real-time data gives insight into the kinetics of protein aggregation and other time-dependent phenomena in a qualitative and quantitative manner. NanoSight has a growing role in biodiagnostics, being proven in detection and speciation of nanovesicles (exosomes) and microvesicles.
NanoSight has installed more than 600 systems worldwide with users including BASF, GlaxoSmithKline, Merck, Novartis, Pfizer, Proctor and Gamble, Roche and Unilever together with the most eminent universities and research institutes. NanoSight's technology is validated by 900+ third party papers citing NanoSight results. NanoSight's leadership position in nanoparticle characterization is consolidated further with publication of an ASTM International standard, ASTM E2834, which describes the NTA methodology for detection and analysis of nanoparticles. For more information, visit www.nanosight.com.